2. 中国科学院亚热带农业生态研究所, 中国科学院亚热带农业生态过程重点实验室, 湖南省畜禽健康养殖工程技术中心, 农业部中南动物营养与饲料科学观测实验站, 长沙 410125;
3. 中国科学院大学, 北京 100049;
4. 长沙兴嘉生物工程股份有限公司, 长沙 410001
2. Scientific Observing and Experimental Station of Animal Nutrition and Feed Science in South-Central of Ministry of Agriculture, Hunan Provincial Engineering Research Center of Healthy Livestock, Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China;
4. Xingjia Bio-Engineering Co., Ltd., Changsha 410001, China
镉(Cadmium,Cd)是自然界存在的天然成分,但是随着在工业和生活中的应用,其在水体和土壤中的含量逐渐增加,其广泛的存在性、强蓄积性和脏器毒性已对人体和畜禽的健康形成了重大的安全隐患[1]。我国饲料卫生标准(GB13078-2001)规定猪、鸡配合饲料中镉浓度不得高于0.5 mg·kg-1。然而在镉污染地区,部分饲料原料中的镉含量远超这个数值[2]。肠道上皮细胞是镉攻击机体的第一道靶点,肝和肾是镉中毒的主要靶点器官[3]。镉可通过多种机制扰乱组织内稳态,首先通过破坏肠道上皮屏障和其紧密连接结构的变化导致肠道结构和功能损伤[4]。镉的肝损伤主要是由于镉通过与肝抗氧化酶中的金属辅基发生竞争性替换,抑制这些酶的活性,造成肝自由基清除能力下降,导致细胞发生脂质过氧化反应和氧化应激[5]。肾镉的蓄积会损伤肾的滤过和重吸收功能[6]。锌和镉属于同族元素,二者的化学性质相似,均以二价阳离子形式存在于生物体内。体内必需金属离子锌离子的含量能够影响镉离子的生物学效应[7]。但是饲料中的植酸类物质易与锌形成动物机体不能吸收的螯合物[8],导致无机锌的利用效率降低。羟基蛋氨酸螯(络)合锌(Methionine hydroxy analog chelated zinc,MHZn)是以1个羟基蛋氨酸分子与1~2个锌原子络合而成的化合物。因为络合物较稳定,能避免肠腔中拮抗因子及其它影响因素对Zn的沉淀和吸附[9],促使更多的Zn到达肠道的吸收位点,从而被机体高效吸收和利用。另外,MHZn可在体内转化为蛋氨酸[10],研究表明蛋氨酸能预防和治疗重金属镉对机体的伤害[11-12]。因此,MHZn不仅具有双重营养的功效,其在缓解镉毒性方面也具有潜在的价值。
本研究利用45日龄仔猪建立镉暴露模型,研究MHZn对镉暴露造成的组织损伤的保护作用及机制,为高效缓解或保护镉暴露造成的机体损伤,以及高效利用微量元素提供了理论依据和技术支持。
1 材料与方法 1.1 试验材料无水氯化镉(纯度99.99%,阿拉丁试剂有限公司)、羟基蛋氨酸锌(含锌12%)由长沙兴嘉生物工程股份有限公司提供。苏木精、伊红染料购自南京雨露实验器材有限公司。所有的化学试剂均为分析纯。
1.2 动物和日粮试验选用24头45日龄的长×大阉公猪,具有相似的初始体重(13.22±1.36) kg,经7 d的适应饲养后,随机分为4个处理组,每个处理组6个重复,每个重复1头猪,单栏饲喂。4个试验组分别为对照组(Con组)、含镉日粮组(30 mg·kg-1,Cd组)、镉(30 mg·kg-1)+低浓度羟基蛋氨酸锌(100 mg·kg-1)日粮组(LMHZn组)、镉(30 mg·kg-1)+高浓度羟基蛋氨酸锌(200 mg·kg-1)日粮组(HMHZn组)。试验期间自由采食和饮水,共30 d。基础日粮参照NRC配合而成,其组成及营养水平见表 1。
|
|
表 1 基础日粮及营养水平 Table 1 Composition and nutrient levels of basal diet |
试验期间每天观察猪生长情况,记录饲料消耗情况,第31天清晨对猪进行空腹称重, 计算仔猪平均日增重、日采食量、料重比,前腔静脉采血5 mL,室温静置1~2 h,3 500 r·m-1离心10 min,取上清液1.5 mL置离心管内,-80 ℃低温冰箱保存待测。屠宰、采样,称量肝、肾、脾重量,计算仔猪脏器系数;取十二指肠、空肠、回肠、肝、肾部分组织迅速放入4%多聚甲醛溶液,摇匀,用于病理切片检测。其余储存于-20 ℃用于其他指标的检测。其中,料重比=平均日采食量(kg)/平均日增重(kg);脏器系数=脏器重量(kg)/仔猪末重(kg)×100%。
1.4 肝、肾、十二指肠、空肠、回肠组织切片的制作将固定的组织样经水洗、透明、浸蜡、包埋等处理后, 在室温下切成5 μm的切片,苏木精-伊红染色,用正置荧光显微镜(BX51,日本奥林巴斯)观察病理变化,测量各肠段的绒毛高度、隐窝深度。参照陈恒灿等[13]的方法处理和观察小肠形态学变化,肝和肾病理学变化观察参照R.E.Dudley等[14]的方法。
1.5 肝、肾中微量元素测定-20 ℃环境中取出仔猪肝、肾,切取0.5 g左右大小的组织块,滤纸吸去表面水分,电子天平准确称量重量后置于一锥形瓶中。向锥形瓶加入20 mL硝酸与高氯酸的混合液(4:1),通风橱内静置过夜充分消化。低火缓慢加热锥形瓶,直至瓶内变透明,液体完全被蒸发。用1%的硝酸溶液重新溶解锥形瓶内剩余物,定容至10 mL后可用于检测。取少量的上样液用电感耦合等离子光谱发生仪(720 ES,安捷伦)检测其中各离子的含量。
1.6 血浆生化指标测定用全自动生化分析仪(cobas c311)进行血浆生化指标的测定。测定内容:白蛋白(Albumin,ALB)、谷丙转氨酶(Alanine aminotransferase,ALT)、谷草转氨酶(Aspartate aminotransferase,AST)、碱性磷酸酶(Alkaline phosphatase,ALP)、乳酸脱氢酶(Lactate dehydrogenase,LDH)、血尿素氮(Blood urea nitrogen,BUN)、血肌酸酐(Creatinine,CREA)、血糖(Blood glucos,GLU)、磷(Phosphorus,P)、高密度脂蛋白(High density lipoprotein,HDL)、低密度脂蛋白(Low-density lipoprotein,LDL)、血氨(Blood ammonia,NH3L)、免疫球蛋白M(Immunoglobulin M,IgM)、总蛋白(Total protein,TP)、二胺氧化酶(Diamine oxidase,DAO)。
1.7 数据处理试验数据采用SPSS17.0统计软件进行单因素方差分析(One-way ANOVA),差异显著时采用Duncan氏法进行组间多重比较,P < 0.05为差异显著,结果以“平均值±标准差”表示。
2 结果 2.1 羟基蛋氨酸锌对镉中毒仔猪生长性能和脏器系数的影响生长性能测定结果显示(表 2),含镉日粮显著降低了仔猪的平均日增重(P < 0.05),提高了料重比(F/G)(P < 0.05)。在含镉日粮中添加MHZn时,仔猪日增重显著升高(P < 0.05),料重比显著降低(P < 0.05),并与Con组无显著差异(P>0.05)。
|
|
表 2 仔猪生长性能和脏器系数 Table 2 Growth performance and organ coefficients of piglets |
脏器系数检测结果显示(表 2),Cd组相较于Con组肝脏系数显著增大(P < 0.05),当MHZn添加量为200 mg·kg-1时,仔猪肝脏系数显著低于Cd组(P < 0.05),且接近Con组水平(P>0.05),各处理组间肾脏系数、脾脏系数无显著差异(P>0.05)。
2.2 羟基蛋氨酸锌对镉中毒仔猪组织病理损伤的影响肠道组织病理学分析结果(图 1B、1F、1J)显示,镉能致十二指肠、空肠损伤,而对回肠的损害作用较小。图 2也表明,相较于Con组,Cd组十二指肠和空肠的绒毛长度显著降低(P < 0.05),隐窝深度显著增高(P < 0.05),绒毛长度/隐窝深度比显著降低(P < 0.05)。LMHZn和HMHZn组相较于Cd组, 十二指肠和空肠绒毛长度显著升高(P < 0.05),空肠隐窝深度显著降低(P < 0.05)。HMHZn组相较于Con组,十二指肠绒毛长度、隐窝深度,空肠隐窝深度、绒毛长度/隐窝深度比无显著差异(P>0.05)。
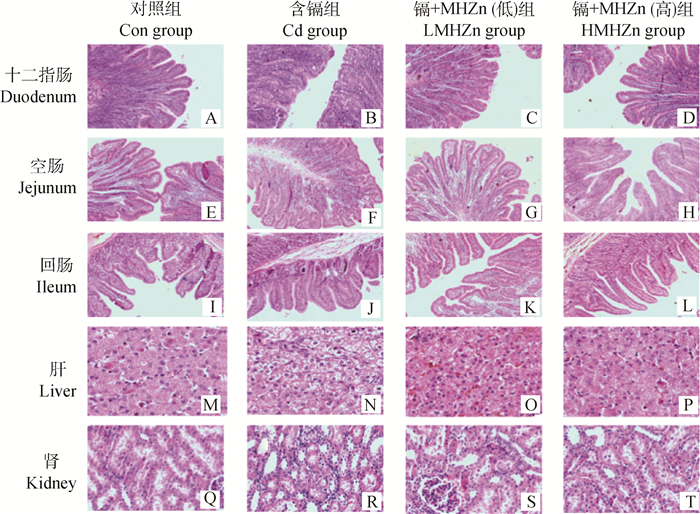
|
图 1 仔猪十二指肠、空肠、回肠、肝、肾病理学分析(100×) Figure 1 Histopathologic analysis of duodenum, jejunum, ileum, liver and kidney in piglets (100×) |
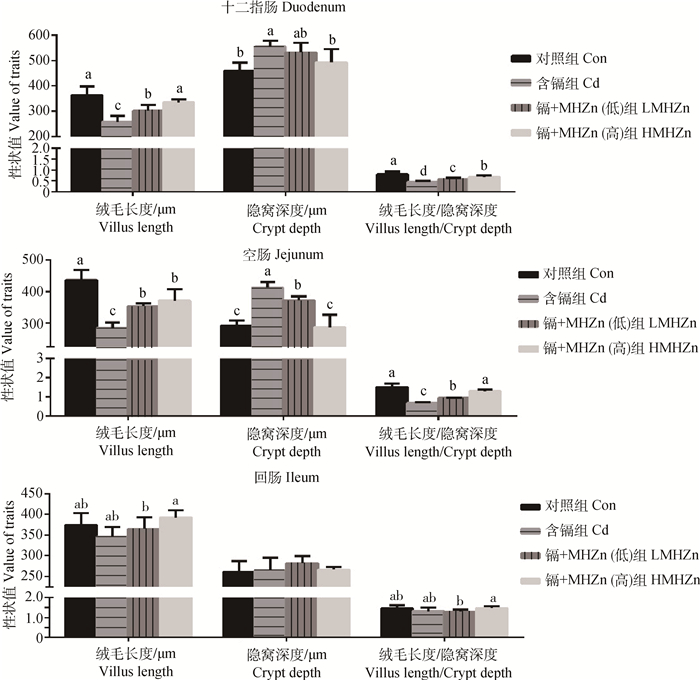
|
图 2 仔猪小肠绒毛长度、隐窝深度、绒毛长度/隐窝深度 Figure 2 Villus length, crypt depth and the ratio of villus height/crypt depth of the small intestine of piglets |
图 1M~1P显示,Cd组仔猪肝细胞肿胀,胞内有空泡,并伴随大范围的颗粒变性和脂肪变性;在含镉日粮中添加MHZn时,可有效缓解上述症状。图 1Q~1T显示,Cd组仔猪的肾小管上皮细胞变性,细胞内可见固缩核;部分肾小管上皮细胞脱落至管腔,并伴有淋巴细胞增生;随着MHZn添加浓度的升高,上述症状逐渐减轻。
2.3 羟基蛋氨酸锌对镉中毒仔猪肝、肾中镉及其他微量元素含量的影响肝中镉和微量元素含量检测结果显示(表 3),Cd组镉、锰含量显著高于Con组(P < 0.05),铁含量显著低于Con组。在含镉日粮中添加MHZn时,与Cd组相比,LMHZn组与HMHZn组镉、锰含量显著降低(P < 0.05)。
|
|
表 3 仔猪肝、肾中镉、铜、铁、镁、锰、锌和钙的含量 Table 3 Concentrations of cadmium, copper, iron, magnesium, manganese, zinc and calcium in liver and kidney of piglets |
肾中镉和微量元素含量检测结果显示(表 3),Cd组镉、铜、锌含量显著高于Con组(P<0.05),铁含量显著低于Con组(P<0.05)。在含镉日粮中添加MHZn,相较于Cd组HMHZn组镉含量显著降低(P<0.05)。
2.4 羟基蛋氨酸锌对镉中毒仔猪血浆生化指标的影响表 4结果显示,与Con组相比,Cd组血浆中ALB含量显著降低(P < 0.05),TP含量呈下降趋势(P>0.05),DAO酶活呈上升趋势(P>0.05)。与Cd组相比,LMHZn组和HMHZn组的ALB和TP有所回升,DAO酶活降低,与对照组无显著差异(P>0.05)。
|
|
表 4 仔猪血浆生化指标的测定结果 Table 4 Analysis of plasma biochemical parameters of piglets |
仔猪镉中毒会导致生长发育迟缓,J. Du等[15]研究表明,(17.25±0.07) kg的仔猪日粮中添加30.49 mg·kg-1的CdCl2,饲喂29 d,仔猪平均日采食量显著降低,料重比显著增高。C.Phillips等[16]研究也发现,断奶30 d的仔猪饲粮中以Cd(NO3)2·4H2O的形式添加2.50 mg·kg-1的镉,3周后仔猪平均日增重,日采食量显著降低。本研究结果显示,仔猪饲喂含镉日粮后,平均日增重显著降低(P<0.05),料重比显著升高(P<0.05),表明镉影响了仔猪的正常生长发育,这一结果与以往报道的一致[15-19]。在含镉日粮中同时添加MHZn时,仔猪日增重显著升高(P<0.05),料重比显著降低(P<0.05),并与对照组无显著差异(P>0.05),表明MHZn可有效减少镉对仔猪的生长抑制作用。
另外,本研究发现,含镉日粮导致仔猪十二指肠和空肠的绒毛长度降低,十二指肠、空肠的隐窝深度增高、绒毛隐窝比减小,这一结果表明,镉导致十二指肠和空肠绒毛上皮细胞的坏死速度超过了更新速度,使得肠道屏障功能受损[20-21]。而MHZn可显著提高小肠绒毛高度(P<0.05),降低隐窝深度(P<0.05),表明MHZn可有效降低镉导致的肠道损伤,有助于维护肠道健康。MHZn的这一功效可能与其分子中的锌密切相关。研究表明,锌具有保护肠黏膜屏障的功能[22-23],在镉暴露条件下补充锌可通过减少肠道镉吸收来降低机体的镉负荷[24]。
肝、肾是镉蓄积的主要器官。镉在肝和肾的过量蓄积会导致组织损伤和细胞的凋亡与坏死[25-26]。韩新艳等研究发现,体重27 kg左右杜长大生长猪饲料中添加10.0 mg·kg-1的镉使肝系数显著提高,肾系数无显著性变化[27],本研究结果与之相符。本研究还发现,含镉日粮在显著提高仔猪肝镉含量的同时,还导致肝细胞肿胀,胞内出现空泡,并伴随大范围的颗粒变性和脂肪变性等病理性损伤,表明肝已出现明显的镉损伤症状[14]。在含镉日粮中添加200 mg·kg-1MHZn时,肝镉含量显著降低,并有效缓解了肝病理学损伤。同时,含镉日粮组仔猪肾镉含量显著增加,并导致部分肾小管上皮细胞变性、脱落,核固缩,淋巴细胞增生。而MHZn可有效缓解肾小管上皮细胞的变性和损伤,减少淋巴细胞的增生,表明MHZn可通过减少肾的镉蓄积,进而减少肾的镉损伤。因此MHZn能够缓解肝肾的镉损伤。
镉作为二价金属离子,与其他二价微量元素,如锌、铜、铁、钙等的物理、化学性质相似,因此镉可与微量元素竞争二价金属离子转运载体,例如二价金属转运蛋白1(DMT1)、膜铁转运蛋白(FPN1,铁和镉共用的转运载体)和ZRT/IRT样蛋白1(ZIP8)等[28]。由于镉与部分转运载体的亲和力高于其它微量元素与转运载体的亲和力,会导致镉被大量吸收,机体出现部分微量元素缺乏的情况[29]。本研究发现,含镉日粮在引起脏器镉蓄积的同时,还导致了肾中铜、锌,肝中锰等二价金属离子的升高,这可能与镉对二价金属离子转运载体的调控以及机体的代偿机制有关。另一方面,含镉日粮降低了肝、肾中铁的含量,表明镉的竞争导致机体对铁的吸收受到抑制。当含镉日粮中添加MHZn(HMHZn组)时,仔猪肝、肾中的镉蓄积显著减少(P<0.05),表明MHZn抑制了机体对镉的吸收与蓄积,有助于减少镉对机体的损伤。同时,由于镉、锌与其他微量元素的竞争关系[30],镉和MHZn的使用影响了其他微量元素的吸收和利用,那么同时添加多种氨基酸螯合微量元素是否更有助于减少镉毒性、维持机体微量元素水平还有待进一步研究。
含镉日粮降低了血浆白蛋白(ALB)和总蛋白(TP)的含量,而血浆ALB和TP的降低,与肝、肾功能异常和消化道疾病密切相关[31],表明镉影响了机体肝、肾和消化道的功能。在含镉日粮中添加MHZn时,镉对ALB和TP的影响有所缓解。二胺氧化酶(DAO)是存在于哺乳动物黏膜或绒毛上层细胞胞浆中一种活性较高的细胞内酶,其活性和黏膜细胞内的核酸和蛋白质合成密切相关。肠黏膜和外周血中DAO活性的高低可有效反映肠上皮细胞的完整性和成熟程度[32]。肠黏膜细胞受损时,DAO酶活升高。本研究发现,含镉日粮导致仔猪DAO酶活升高,表明肠黏膜存在一定的损伤,而HMHZn组的DAO酶活显著低于Cd组(P<0.05),进一步表明MHZn有助于减少镉对肠黏膜的损伤。
4 结论本研究结果表明,日粮添加100和200 mg·kg-1的MHZn可有效减少含镉饲料对仔猪的影响,提高仔猪的生长性能,减少肝、肾中的镉蓄积及镉的致毒性,显著降低镉对小肠绒毛的损伤。
| [1] | ZHANG Y F, LIU P, WANG C N, et al. Human health risk assessment of cadmium via dietary intake by children in Jiangsu Province, China[J]. Environ Geochem Health, 2017, 39(1): 29–41. DOI: 10.1007/s10653-016-9805-5 |
| [2] |
金秀娥, 舒金秀. 饲料中镉的危害与防治[J]. 中国农业科技导报, 2009, 11(S1): 6–9.
JIN X E, SHU J X. Hazards and prevention to cadmium in feed[J]. Journal of Agricultural Science and Technology, 2009, 11(S1): 6–9. (in Chinese) |
| [3] | NORDBERG G F, NOGAWA K, NORDBERG M. Cadmium[M]//NORDBERG G F, FOWLER B A, NORDBERG M. Handbook on the Toxicology of Metals. 4th ed. New York: Academic Press, 2015: 667-716. |
| [4] | KIM E, LEMBERT M M, OPIYO S O, et al. Differential role of oxidative stress pathways and microbiota in the development of allergen specific IgE following chronic ingestion of low doses of cadmium[J]. J Immunol, 2017, 198(S): 194.5. |
| [5] |
张鼎. 镉中毒及锌对镉中毒的作用机理研究[D]. 武汉: 华中农业大学, 2015.
ZHANG D. Cadmium poisoning and effect of zinc supplement on cadmium cytotoxicity[D]. Wuhan: Huazhong Agricultural University, 2015. (in Chinese) |
| [6] | CHEN X C, LI J, CHENG Z W, et al. Low dose cadmium inhibits proliferation of human renal mesangial cells via activation of the JNK pathway[J]. Int J Environ Res Public Health, 2016, 13(10): 990. |
| [7] | MOULIS J M. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals[J]. BioMetals, 2010, 23(5): 877–896. DOI: 10.1007/s10534-010-9336-y |
| [8] |
于昱, 吕林, 张亿一, 等. 影响动物肠道锌吸收因素的研究进展[J]. 动物营养学报, 2007, 19(S1): 459–464.
YU Y, LV L, ZHANG Y Y, et al. Research advances on factors of affecting zinc intestinal absorption[J]. Chinese Journal of Animal Nutrition, 2007, 19(S1): 459–464. (in Chinese) |
| [9] |
赵士堂. 羟基蛋氨酸锌在奶牛上的应用研究[D]. 杭州: 浙江大学, 2003.
ZHAO S T. Study of zinc hydroxy-methionine on dairy cows[D]. Hangzhou: Zhejiang University, 2003. (in Chinese) |
| [10] | SUN Q J, GUO Y M, LI J H, et al. Effects of methionine hydroxy analog chelated Cu/Mn/Zn on laying performance, egg quality, enzyme activity and mineral retention of laying hens[J]. J Poult Sci, 2012, 49(1): 20–25. DOI: 10.2141/jpsa.011055 |
| [11] | PRAKASH R. Influence of methionine on cadmium toxicity[J]. Adv Pharmacol Toxicol, 2013, 14(1): 7–9. |
| [12] | GUBRELAY U, MATHUR R, KANNAN G M, et al. Role of S-adenosyl-L-methionine in potentiating cadmium mobilization by diethylenetriamine penta acetic acid in mice[J]. Cytobios, 2001, 104(406): 99–105. |
| [13] |
陈恒灿, 马黎, 陈克嶙, 等. 丙氨酰谷氨酰胺二肽对早期断奶仔猪抗氧化能力及肠道保护的研究[J]. 畜牧兽医学报, 2011, 42(2): 251–259.
CHEN H C, MA L, CHEN K L, et al. Studies on alany-glutamine on antioxidant ability and protects of small intestine for early weanling piglets[J]. Acta Veterinaria et Zootechnica Sinica, 2011, 42(2): 251–259. (in Chinese) |
| [14] | DUDLEY R E, SVODOBA D J, KLAASSEN C D. Acute exposure to cadmium causes severe liver injury in rats[J]. Toxicol Appl Pharmacol, 1982, 65(2): 302–313. DOI: 10.1016/0041-008X(82)90013-8 |
| [15] | DU J, CHENG S Y, HOU W X, et al. Effectiveness of maifanite in reducing the detrimental effects of cadmium on growth performance, cadmium residue, hematological parameters, serum biochemistry, and the activities of antioxidant enzymes in pigs[J]. Biol Trace Elem Res, 2013, 155(1): 49–55. DOI: 10.1007/s12011-013-9769-6 |
| [16] | PHILLIPS C, GYÖRI Z, KOVÁCS B. The effect of adding cadmium and lead alone or in combination to the diet of pigs on their growth, carcase composition and reproduction[J]. J Sci Food Agric, 2003, 83(13): 1357–1365. DOI: 10.1002/jsfa.v83:13 |
| [17] | HOOGENBOOM R L A P, HATTINK J, VAN POLANEN A, et al. Carryover of cadmium from feed in growing pigs[J]. Food Addit Contam A, 2015, 32(1): 68–79. DOI: 10.1080/19440049.2014.979370 |
| [18] | HAN X Y, XU Z R, WANG Y Z, et al. Effect of cadmium on lipid peroxidation and activities of antioxidant enzymes in growing pigs[J]. Biol Trace Elem Res, 2006, 110(3): 251–263. DOI: 10.1385/BTER:110:3 |
| [19] | NWOKOCHA C R, OWU D U, UFEARO C S, et al. Comparative study on the efficacy of Garcinia kola in reducing some heavy metal accumulation in liver of Wistar rats[J]. J Ethnopharmacol, 2011, 135(2): 488–491. DOI: 10.1016/j.jep.2011.03.049 |
| [20] | ZHAO Z H, HYUN J S, SATSU H, et al. Oral exposure to cadmium chloride triggers an acute inflammatory response in the intestines of mice, initiated by the over-expression of tissue macrophage inflammatory protein-2 mRNA[J]. Toxicol Lett, 2006, 164(2): 144–154. DOI: 10.1016/j.toxlet.2005.12.004 |
| [21] | DUIZER E, GILDE A J, VERSANTVOORT C H M, et al. Effects of cadmium chloride on the paracellular barrier function of intestinal epithelial cell lines[J]. Toxicol Appl Pharmacol, 1999, 155(2): 117–126. DOI: 10.1006/taap.1998.8589 |
| [22] | ZHOU Z. Zinc and alcoholic liver disease[J]. Digest Dis, 2010, 28(6): 745–750. DOI: 10.1159/000324282 |
| [23] | ZHOU X, LI Y S, LI Z J, et al. Effect of dietary zinc on morphological characteristics and apoptosis related gene expression in the small intestine of Bama miniature pigs[J]. Acta Histochem, 2017, 119(3): 235–243. DOI: 10.1016/j.acthis.2017.01.006 |
| [24] | ZHONG W, LI Q, SUN Q, et al. Preventing gut leakiness and endotoxemia contributes to the protective effect of zinc on alcohol-induced steatohepatitis in rats[J]. J Nutr, 2015, 145(12): 2690–2698. DOI: 10.3945/jn.115.216093 |
| [25] | NORDBERG G F. Historical perspectives on cadmium toxicology[J]. Toxicol Appl Pharmacol, 2009, 238(3): 192–200. DOI: 10.1016/j.taap.2009.03.015 |
| [26] | JIHEN E H, FATIMA H, NOUHA A, et al. Cadmium retention increase:a probable key mechanism of the protective effect of zinc on cadmium-induced toxicity in the kidney[J]. Toxicol Lett, 2010, 196(2): 104–109. DOI: 10.1016/j.toxlet.2010.04.006 |
| [27] |
韩新燕, 许梓荣, 李红文, 等. 饲料中加镉对猪生长性能及内脏器官的影响[J]. 中国粮油学报, 2005, 20(2): 76–79.
HAN X Y, XU Z R, LI H W, et al. Effects of dietary cadmium on growth performance and relative weight of organs in growing pigs[J]. Journal of the Chinese Cereals and Oils Association, 2005, 20(2): 76–79. (in Chinese) |
| [28] | WANG Y H, ZALUPS R K, BARFUSS D W. Potential mechanisms involved in the absorptive transport of cadmium in isolated perfused rabbit renal proximal tubules[J]. Toxicol Lett, 2010, 193(1): 61–68. DOI: 10.1016/j.toxlet.2009.12.007 |
| [29] | VESEY D A. Transport pathways for cadmium in the intestine and kidney proximal tubule:focus on the interaction with essential metals[J]. Toxicol Lett, 2010, 198(1): 13–19. DOI: 10.1016/j.toxlet.2010.05.004 |
| [30] | BRIDGES C C, ZALUPS R K. Molecular and ionic mimicry and the transport of toxic metals[J]. Toxicol Appl Pharmacol, 2005, 204(3): 274–308. DOI: 10.1016/j.taap.2004.09.007 |
| [31] | TENNANT C J. Potential contribution of dietary sources to urinary cadmium and β2-microglobulin excretion of occupationally exposed workers[J]. J Occup Med, 1991, 33(11): 1175–1179. DOI: 10.1097/00043764-199111000-00016 |
| [32] | TUFVESSON G, TRYDING N. Determination of diamine oxidase activity in normal human blood serum[J]. Scand J Clin Lab Invest, 1969, 24(2): 163–168. DOI: 10.3109/00365516909080147 |



