2. 洛阳师范学院生命科学学院, 洛阳 471022;
3. 南京农业大学动物医学院农业部动物细菌学重点实验室, 南京 210095
2. College of Life Science, Luoyang Normal University, Luoyang 471022, China;
3. Key Lab of Animal Bacteriology of Ministry of Agriculture, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China
密度感应系统(quorum sensing,QS)指的是菌体随着群体数量增大,细菌个体之间会通过分泌一些小分子物质来进行细菌间交流,从而控制细菌基因表达的相关机制。J. Engebrecht等在研究费氏弧菌密度依赖性生物发光时第一次发现了QS,研究发现诱导生物发光是由细菌产生的小信号化合物的浓度依赖性作用介导的,称为自诱导分子(autoinducer,AI)[1-2]。随后在30多种革兰阴性细菌中,均发现存在类似的系统,其对多种细胞密度依赖性过程进行控制。QS在各类细菌中的代表系统包括以下几种:革兰阴性菌(G-)的LuxR-LuxI信号系统;革兰阳性菌(G+)的自诱导信号分子(autoinducing peptide, AIP)信号系统;G+和G-共同存在的LuxS/AI-2系统等[3]。QS系统目前呈现复杂性和多样性两种明显的特点。复杂性来源于信号分子的多功能性、多种信号分子具有相同的功能性、QS系统组成的复杂性以及多种QS系统之间交流的复杂性。多样性体现在分布多样性、信号分子多样性、信号分子产生机制多样性、信号分子运输多样性和信号分子响应多样性。大量研究表明QS可以调节多种生理功能,其中就包含菌体发光、抗生素合成、质粒转移、生物被膜(biofilm, BF)形成等[4]。猪链球菌(Streptococcus suis,SS)作为普遍存在的人兽共患病原菌,对养猪业造成严重危害,尤其是在东亚及东南亚国家,比如泰国、越南、中国等[5-7]。猪链球菌病作为一种全球性传染病,已经被报道在30个国家/地区蔓延,不少于1 600人被感染,其中有些已死亡,严重威胁着人类健康和养猪产业的发展[8]。SS的QS系统是其产生毒力和耐药性的主要因素之一,目前研究最热的就是猪链球菌LuxS/AI-2型QS系统。
1 QS的一般机制QS每种系统都存在相应的小分子物质,这些物质统称为细菌信息素(bacterial pheromones),即自身诱导分子AI,这些生物小分子的浓度与细菌密度呈正相关[9-11]。目前,发现的QS信号分子可分为:G-的QS系统的信号分子酰基高丝氨酸内酯类自诱导分子(acyl-homosefine lactone,AHL),这类信号分子的头部均为高丝氨酸内酯环,存在差异的部分一般为酰基侧链尾巴,推测是取代基和侧链长短不同造成的,这就导致细菌在利用信号分子时存在特异性,而且AHL能够穿过细菌生物被膜,并在细菌外形成聚集;G+的AIP,该信号分子在不同的细菌中的结构有所不同,大部分AIP分子的氨基酸残基界于5~17之间[12]。AIP不能单独通过细胞壁,一般需要ABC转运系统(ATP-binding-cassette)或其他膜通道蛋白协助转运至膜外发挥作用[13]。菌体的二元信号系统通过识别菌体外的AIP分子,并经过一个复杂的信号转导过程,最终调控靶基因的转录表达;G+和G-共有的AI-2信号分子介导的QS的细菌间信息交流方式,由于AI-2的合成需要LuxS蛋白酶的参与,因此该过程依赖于luxS基因[14-19]。除上述几种信号分子以外,研究发现喹啉酮类化合物、脂肪酸和部分酯类化合物也可作为密度感应系统的信号分子[20]。
2 LuxS/AI-2型密度感应系统B. L. Bassler等[21]在研究哈维氏弧菌的发光原理时发现S-核糖基高半胱氨酸酶由luxS基因编码产生。该酶分解形成同型半胱氨酸和4,5-二羟基-2,3-戊二酮(4, 5-dihydroxy-2, 3-pentanedione, DPD),DPD通过自环化形成AI-2,结构为呋喃酮酰硼酸二酯,这类信号分子的共同点就是它们的前体均是DPD[22-23]。AI-2在活性甲基循环(activated methylcycle,AMC)中具有平衡代谢的作用,起初,普遍认为AI分子只能够在同种细菌间发挥作用,但是在研究哈氏弧菌的QS时发现了不同于传统的细菌QS调节通路[24-27]。随着细菌密度的增高,AI-2介导的QS激活,LuxO磷酸化终止,不再抑制LuxCDABE操纵子,并且LuxO与LuxR共同作用使LuxCDABE操纵子转录,从而使细菌发光,这就称为LuxS/AI-2型QS。目前已明确存在luxS基因类似物的菌种超过55种,但明确AI-2分子结构的只有很少一部分。
韩先干等[28]和M. Cao等[29]对SS基因组分析表明,SS中存在的luxS基因,可以产生AI-2信号分子,将SS2的luxS基因序列与哈维氏弧菌的luxS基因序列进行对比,结果显示一致性为36%,相似性为56%。将其与其他种类链球菌的luxS基因进行比较,则具有80%以上的相似性,表明细菌中的luxS基因属于高度保守性基因。
3 LuxS蛋白的结构和活性LuxS属于一种小型金属酶,所含氨基酸的数目在170个左右。LuxS蛋白为一个二级结构,该结构由四个反平行的β折叠和四个反平行的α螺旋组成[30-33]。在观察这些基因的结构时,发现该折叠晶体高度保守,可能在α-β家族的折叠是一个新的类型。研究分析LuxS蛋白时发现其保守的基序为His-Xaa-Xaa-Glu-His。通过电子杂交分析哈维氏弧菌的LuxS蛋白,发现该菌的基序为Lys-Ile-Pro-Glu-Leu-Asn-Glu-Tyr[34]。
本课题组获得了猪链球菌LuxS蛋白晶体(图 1),解析了其三维精细结构(PDB索引号为4XCH),研究发现每个晶体不对称单元里有4个LuxS单体蛋白。单体LuxS蛋白同样是由四个反平行的β折叠和四个反平行的α螺旋组成,顺序是H1-S1-S2-H2-S3-S4-H3-H4。等离子耦合质感质谱可知Zn2+离子是猪链球菌LuxS蛋白活性中心的主要成分,试验结果和S. N. Ruzheinikov的报道[35]一致,但是R. Rajan等[33]研究发现枯草芽胞杆菌的LuxS蛋白中存在Fe2+,提示不同细菌LuxS蛋白活性中心附件的金属离子可能有差异,并推测这可能与它的催化效率有关。生物信息学分析发现位于底物结合位点附近80和87位氨基酸发生了可能的进化突变,通过定点突变发现2个氨基酸的突变对其底物结合和酶的催化能力有较大影响,并影响猪链球菌AI-2的产生和BF的形成能力。体内体外试验证明,这两个氨基酸的缺乏或变异能够抑制AI-2分子的产生和BF的形成[36-37]。
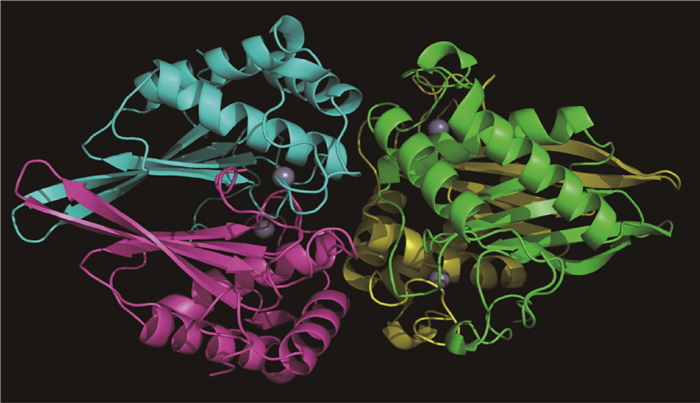
|
球体表示Zn2+;LuxS单体蛋白用不同颜色表示 Zn2+ ions are represented by spheres; LuxS monomeric proteins are showed by different colors 图 1 SS2 HA9801株LuxS蛋白晶体结构图 Figure 1 LuxS protein crystal structure of SS2 HA9801 |
在细菌基因表达的主要功能中,LuxS循环途径是一个重要组成部分。在功能上,主要是负责水解S-腺苷同型半胱氨酸,然后回收代谢水解产物S-腺苷甲硫氨酸(S-adenosylmethionine, SAM)。SAM途径是细菌甲基再循环的主要方式,也是细菌多胺形成和维生素合成的关键[38]。luxS基因的突变或缺失将会导致SAM功能缺失和AI-2合成的受阻,这个现象表明,如果luxS突变导致相关表型出现差异,那么QS活动就会受到影响。另外,诱变luxS基因也会导致SAM其他代谢产物在细胞外浓度的变化[39]。利用重组LuxS蛋白检测,已经证明,在常规luxS缺陷性活菌株的培养物上清液中,S-核糖基高半胱氨酸(具有LuxS功能的SAM中间体)的细胞外浓度显著增加[40]。基于以上研究结果,许多存在于luxS突变株细胞内和细胞外的SAM通路中间体浓度都在改变是有可能的。luxS突变细菌对SAM代谢产物的回收产生显著影响。
5 LuxS/AI-2调控猪链球菌相关网络LuxS/AI-2系统是细菌全局调控网络的一部分,对细菌种群密度变化波动作出反应,调控不同基因的表达,从而调节细菌的生长和毒力等多种生命活动,使其适应不同的环境[41-44]。在复杂的调控网络中,AI-2除了有正调控作用以外还有负调控作用。目前,AI-2分子的产生在细菌中已经得到了广泛的发现,但其完整的感应通路及调控方式尚不完全明确。寻找细菌AI-2分子的受体蛋白及研究其介导的下游调控网络是当前研究的热点方向,但是目前在猪链球菌上还无实质性进展。本课题组构建了猪链球菌Tn917转座子随机突变库,力求筛选鉴定出AI-2的受体基因,并通过基因芯片和蛋白组学获知AI-2及其受体基因介导的下游调控网络,目前也取得了一定的进展。
5′-甲硫腺苷/S-腺苷高半胱氨酸核苷酶(5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase,Pfs)在细菌代谢调控方面具有重要作用,与LuxS酶共同促进AI-2分子在细菌体内的合成。在SS2对数生长后期,AI-2的活性达到最高,同时,也是pfs基因转录水平达到最高的时期,相比之下,luxS基因转录水平达到最高的时期处于SS2的稳定期[45]。通过Agilent基因芯片,比较SS2的野生菌株和ΔluxS株基因在转录表达水平的差异,结果发现缺失株的312个基因与野生株出现差异表达,其中上调基因为144个,下调基因有168个。通过在SS2 ΔluxS培养基中体外添加DPD,结果发现DPD能够引起71个基因的差异表达,其中29个基因被认为是受LuxS/AI-2密度感应系统调控的靶基因,这些基因与细菌毒力及铁的摄取有关[27]。本课题组构建了luxS+过表达菌株,通过Real-time PCR证实SS在各个时期luxS基因表达量都有提高,而pfs基因水平维持不变,过表达菌株并不能增加AI-2分子的产生水平[46]。分析原因可能是AI-2的产生是由luxS基因和pfs基因共同作用产生,只增加LuxS蛋白并不会提高AI-2的产生水平。
6 LuxS/AI-2系统对SS生物学特性的影响LuxS/AI-2系统影响细菌生物学特性是复杂而有序的,作为重要的细菌全局调控网络之一,通过对细菌数量变化浮动作出应答,调控相应基因的表达,从而影响各种生命活动。通常调控一种基因往往会影响多种表型的变化,这说明某一种基因的改变,会影响多个相关基因的表达。现已发现细菌的许多生理功能都受LuxS/AI-2系统的调节,包括细菌发光、抗生素敏感性、运动、质粒的转移、毒力、基因的表达、生物被膜形成等[47-49]。关于LuxS/AI-2对SS生物学特性的调控目前研究主要包括以下几个方面。
6.1 细菌生长和菌落形态观察比较细菌突变株与野生株的生长曲线和形态变化,可以更直观地了解突变株的生理变化。通过比较SS2野生菌株和ΔluxS株的生长曲线,发现缺失株的生长速度低于野生菌株,其对数期相对滞后,数量增加的速度也低于野生菌株,野生株提前90 min到达静止期[50]。在普通显微镜下SS2野生菌株的形态为标准的SS形态,ΔluxS株菌体在显微镜下呈现聚集状态,形成的链长也明显短于野生菌株,在电镜下观察两种菌株的形态,发现缺失株的荚膜厚度变薄,且对H2O2的耐受性增强[27]。
6.2 BF形成细菌BF是一种由细菌分泌并包裹在细菌群体外的一种区别于细菌荚膜的膜样物,BF在细菌致病方面有着很大的作用,也是使细菌出现耐药性的重要原因之一[51]。本课题组研究发现给SS2培养基中添加0~2 μmol·L-1的AI-2分子,会显著提高细菌形成BF的能力,但当外源性AI-2添加的量为2~15 μmol·L-1时,SS2形成BF的能力受到抑制。当以添加2 μmol·L-1 AI-2分子作为基础研究最佳作用时间时,发现在24 h添加该信号分子可显著增加BF的形成,在48 h添加无影响[52]。对ΔluxS株进行测试发现在24、48 h均能够显著增加BF形成,过表达试验发现SS2的BF形成能力增强,且这种能力随着培养时间的延长有所加强[46]。
6.3 黏附力致病菌感染宿主的第一步通常为黏附、定植,致病菌黏附力的大小决定了其致病性的强弱[53]。通过研究SS2ΔluxS株与野生菌株黏附力的差异,将有利于研究LuxS/AI-2系统的相关调控机制。通过观察比较SS2野生菌株和ΔluxS株对人上皮样喉癌细胞株Hep-2和人脐静脉内皮细胞HUVEC的黏附能力,发现ΔluxS株相比于野生菌株,对两种细胞的黏附能力均下降。本课题组分别在SS2野生株和ΔluxS株培养液中加入外源的AI-2,结果发现,低浓度添加AI-2会增强细菌对细胞的黏附,高浓度则会降低这种黏附能力,4和6 μmol·L-1是其最佳AI-2添加量[51]。
6.4 细菌毒力细菌毒力指的是病原细菌致病能力的强弱程度[54]。致病菌都含有大量产生毒力因子的相关基因,关于LuxS在细菌毒力方面的作用已有研究,并显示该基因在调控细菌毒力方面起重要作用[55]。本课题组通过实时PCR定量发现,luxS基因的缺失能够导致毒力基因——谷氨酸脱氢酶基因(gdh)、荚膜多糖基因(cps)、溶菌酶释放蛋白基因(mrp)、甘油醛-3-磷酸脱氢酶基因(gapdh)、猪溶血素基因(sly)、纤黏连蛋白基因(fbps)和细胞外蛋白因子基因(ef)分别下降0.66、0.61、0.45、0.48、0.29、0.57和0.38。斑马鱼感染试验发现ΔluxS株毒力下降了10倍,互补株能够恢复部分毒力[37],猪体的感染试验也获得了类似的结果,缺失株在猪肺、脑、关节等各器官中细菌数量均明显低于野生株[27]。
6.5 溶血活性红细胞溶血会造成机体出现贫血、败血症等症状,检测细菌溶血活性是检验其致病力大小的重要指标[56]。通过检测SS2 HA9801野生株、ΔluxS株和C-ΔluxS互补株的溶血活性,发现三者能够裂解50%红细胞的最大稀释倍数分别为1:16、1:2和1:16,说明luxS基因能够影响细菌的溶血能力[55]。
7 针对LuxS/AI-2型密度感应系统功能制定的抗菌策略LuxS/AI-2在细菌交流、BF形成、代谢和毒力方面的重要作用,提示了一种新型抗菌策略,即设计干扰致病菌LuxS/AI-2系统的新药物,以达到控制病菌的目的[57-60]。目前已尝试的方法包括阻断AI-2产生,本课题组通过噬菌体展示技术筛选到一个能特异性阻断AI-2产生的多肽,破坏由QS调控的猪链球菌毒力因子的表达,显著降低猪链球菌在机体内的侵染能力[61-64]。另一个为抑制AI-2分子信号功能,即设计AI-2分子类似物,与受体蛋白竞争性结合来阻断AI-2介导的信号通路[65]。目前已经有学者成功干扰了信号分子与受体蛋白的结合。这种干扰抑制了病原菌在动物体内的毒力,而人类没有luxS基因,对人类没有副作用。相对于传统的抗生素而言,QS抑制剂不仅阻止细菌生长或者将其杀死,而且不会导致细菌耐药株的产生。因此,抗QS信息传导的化合物在抑制细菌的感染方面具有巨大的研究价值。
8 展望LuxS/AI-2型QS系统能够通过调整自然环境下细菌间信息交流因子,调控菌群生物学特性。对于病原性细菌,认识和掌握LuxS/AI-2型QS系统,进而打开新的抗菌思路具有重要意义。因此深入研究获得参与SS对AI-2摄取的基因,哪些是与AI-2具有结合活性呢?AI-2是通过调控SS的哪些靶蛋白来调控SS的生物学特性?AI-2结合受体蛋白后调控的下游靶蛋白有哪些,如何调控其QS信号通路的?详细阐明细菌对AI-2的摄取及其介导的密度感应系统的分子调控网络机制,以期通过抑制病原菌信号传导来达到控制细菌的目的。可以预见,对于致病菌的防控方面,QS是具有研究和应用前景的新药靶标。
| [1] | ENGEBRECHT J, NEALSON K, SILVERMAN M. Bacterial bioluminescence:isolation and genetic analysis of functions from Vibrio fischeri[J]. Cell, 1983, 32(3): 773–781. DOI: 10.1016/0092-8674(83)90063-6 |
| [2] | ZHAO W N, LORENZ N, JUNG K, et al. Fimbrolide natural products disrupt bioluminescence of Vibrio by targeting autoinducer biosynthesis and luciferase activity[J]. Angew Chem Int Ed Engl, 2016, 55(3): 1187–1191. DOI: 10.1002/anie.201508052 |
| [3] |
徐翔. 外源AHL对中慢生型天山根瘤菌群体感应系统作用的初步研究及其对根瘤菌根际定殖能力的影响[D]. 南京: 南京农业大学, 2008.
XU X. Study on foreign AHL's effect on the quorum sensing system of M. tianshanesne and the function of root colonization[D]. Nanjing: Nanjing Agricultural University, 2008. (in Chinese) http://kns.cnki.net/KCMS/detail/detail.aspx?filename=tura200901013&dbname=CJFD&dbcode=CJFQ |
| [4] | ZHENG H M, MAO Y L, ZHU Q C, et al. The quorum sensing regulator CinR hierarchically regulates two other quorum sensing pathways in ligand-dependent and -independent fashions in Rhizobium etli[J]. J Bacteriol, 2015, 197(9): 1573–1581. DOI: 10.1128/JB.00003-15 |
| [5] | HATRONGJIT R, KERDSIN A, GOTTSCHALK M, et al. First human case report of sepsis due to infection with Streptococcus suis serotype 31 in Thailand[J]. BMC Infect Dis, 2015, 15: 392. DOI: 10.1186/s12879-015-1136-0 |
| [6] | WERTHEIM H F L, NGUYEN H N, TAYLOR W, et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam[J]. PLoS One, 2009, 4(6): e5973. DOI: 10.1371/journal.pone.0005973 |
| [7] | WANG S J, GAO M, AN T, et al. Genetic diversity and virulence of novel sequence types of Streptococcus suis from diseased and healthy pigs in China[J]. Front Microbiol, 2015, 6: 173. |
| [8] | CHATZOPOULOU M, VOULGARIDOU I, PAPALAS D, et al. Third case of Streptococcus suis infection in Greece[J]. Case Rep Infect Dis, 2015, 2015: 505834. |
| [9] | WELSH M A, EIBERGEN N R, MOORE J D, et al. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes[J]. J Am Chem Soc, 2015, 137(4): 1510–1519. DOI: 10.1021/ja5110798 |
| [10] | CHANG J C, JIMENEZ J C, FEDERLE M J. Induction of a quorum sensing pathway by environmental signals enhances group A streptococcal resistance to lysozyme[J]. Mol Microbiol, 2015, 97(6): 1097–1113. DOI: 10.1111/mmi.2015.97.issue-6 |
| [11] | WANG B Y, ZHAO A S, XIE Q, et al. Functional plasticity of the AgrC receptor histidine kinase required for staphylococcal virulence[J]. Cell Chem Biol, 2017, 24(1): 76–86. DOI: 10.1016/j.chembiol.2016.12.008 |
| [12] | MILLER M B, BASSLER B L. Quorum sensing in bacteria[J]. Annu Rev Microbiol, 2001, 55: 165–199. DOI: 10.1146/annurev.micro.55.1.165 |
| [13] | ZOLLMANN T, MOISET G, TUMULKA F, et al. Single liposome analysis of peptide translocation by the ABC transporter TAPL[J]. Proc Natl Acad Sci U S A, 2015, 112(7): 2046–2051. DOI: 10.1073/pnas.1418100112 |
| [14] | PLYUTA V A, LIPASOVA V A, KOKSHAROVA O A, et al. The effect of introduction of the heterologous gene encoding the N-acyl-homoserine lactonase (aiiA) on the properties of Burkholderia cenocepacia 370[J]. Genetika, 2015, 51(8): 864–872. |
| [15] | TRAPPETTI C, MCALLISTER L J, CHEN A, et al. Autoinducer 2 signaling via the phosphotransferase FruA drives galactose utilization by Streptococcus pneumoniae, resulting in hypervirulence[J]. MBio, 2017, 8(1): e02269–16. |
| [16] | MA R H, QIU S W, JIANG Q, et al. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus[J]. Int J Med Microbiol, 2017, 307(4-5): 257–267. DOI: 10.1016/j.ijmm.2017.03.003 |
| [17] | ZHU S Q, WU H H, ZENG M Y, et al. Regulation of spoilage-related activities of Shewanella putrefaciens and Shewanella baltica by an autoinducer-2 analogue, (Z)-5-(Bromomethylene) furan-2(5H)-one[J]. J Food Process Preserv, 2015, 39(6): 719–728. DOI: 10.1111/jfpp.12281 |
| [18] | NOVOTNY L A, JURCISEK J A, WARD M O Jr, et al. Antibodies against the majority subunit of type Ⅳ pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media[J]. Mol Microbiol, 2015, 96(2): 276–292. DOI: 10.1111/mmi.2015.96.issue-2 |
| [19] | LIM J, LEE K M, PARK C Y, et al. Quorum sensing is crucial to Escherichia coli O157:H7 biofilm formation under static or very slow laminar flow conditions[J]. BioChip J, 2016, 10(3): 241–249. DOI: 10.1007/s13206-016-0310-9 |
| [20] |
楼秀余. 脂肪酸乙醇胺类衍生物分子的生物学功能[J]. 中国科技博览, 2015(42): 274.
LOU X Y. The biological function of fatty acid ethanolamine derivatives[J]. China Science and Technology Review, 2015(42): 274. (in Chinese) |
| [21] | BASSLER B L, WRIGHT M, SHOWALTER R E, et al. Intercellular signalling in Vibrio harveyi:sequence and function of genes regulating expression of luminescence[J]. Mol Microbiol, 1993, 9(4): 773–786. DOI: 10.1111/mmi.1993.9.issue-4 |
| [22] | SINTIM H O, BENTLEY W E, ROY V, et al. Phosphorylated and branched dihydroxy-pentane-dione (DPD) analogs as quorum sensing inhibitors in bacteria: US, US8952192(B2)[P]. 2015-02-10. |
| [23] | ARNOLD W K, SAVAGE C R, ANTONICELLO A D, et al. Apparent role for Borrelia burgdorferi LuxS during mammalian infection[J]. Infect Immun, 2015, 83(4): 1347–1353. DOI: 10.1128/IAI.00032-15 |
| [24] | MITRA A, HERREN C D, PATEL I R, et al. Integration of AI-2 based cell-cell signaling with metabolic cues in Escherichia coli[J]. PLoS One, 2016, 11(6): e0157532. DOI: 10.1371/journal.pone.0157532 |
| [25] | QUAN D N, TSAO C Y, WU H C, et al. Quorum sensing desynchronization leads to bimodality and patterned behaviors[J]. PLoS Comput Biol, 2016, 12(4): e1004781. DOI: 10.1371/journal.pcbi.1004781 |
| [26] | COLLINS K C, TSUCHIKAMA K, LOWERY C A, et al. Dissecting AI-2-mediated quorum sensing through C5-analogue synthesis and biochemical analysis[J]. Tetrahedron, 2015, 72(25): 3593–3598. |
| [27] | XU F, SONG X N, CAI P J, et al. Quantitative determination of AI-2 quorum-sensing signal of bacteria using high performance liquid chromatography-tandem mass spectrometry[J]. J Environ Sci, 2017, 52: 204–209. DOI: 10.1016/j.jes.2016.04.018 |
| [28] |
韩先干. 猪链球菌2型LuxS/AI-2型密度感应系统研究[D]. 南京: 南京农业大学, 2008.
HAN X G. Study on the quorum sensing of LuxS/AI-2 of Streptococcus suis serotype 2[D]. Nanjing: Nanjing Agricultural University, 2008. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10307-1012271423.htm |
| [29] | CAO M, FENG Y J, WANG C J, et al. Functional definition of LuxS, an autoinducer-2(AI-2) synthase and its role in full virulence of Streptococcus suis serotype 2[J]. J Microbiol, 2011, 49(6): 1000–1011. DOI: 10.1007/s12275-011-1523-1 |
| [30] | YUVARANI T, ANURADHA V, YOGANANTH N, et al. Molecular modelling and structure analysis of S-ribosyl homocysteinase from Aeromonas hydrophila[J/OL]. Biosci Biotechnol Res Asia, 2014, 11: 363-368. [2017-09-29]. http://www.biotech-asia.org/vol11_nospl_edn1/molecular-modelling-and-structure-analysis-of-s-ribosyl-homocysteinase-from-aeromonas-hydrophila/ |
| [31] |
马艳平, 张杰, 陈豪泰, 等. 副猪嗜血杆菌S-核糖基高半胱氨酸酶基因序列分析与推导蛋白三维结构的分子模拟[J]. 中国兽医学报, 2011, 31(1): 40–44.
MA Y P, ZHANG J, CHEN H T, et al. Sequence analysis of luxS gene of Haemophilus parasuis and homology modeling of 3D structure of the deduced protein[J]. Chinese Journal of Veterinary Science, 2011, 31(1): 40–44. (in Chinese) |
| [32] | BHATTACHARYYA M, VISHVESHWARA S. Functional correlation of bacterial LuxS with their quaternary associations:interface analysis of the structure networks[J]. BMC Struct Biol, 2009, 9(1): 8. DOI: 10.1186/1472-6807-9-8 |
| [33] | RAJAN R, ZHU J E, HU X, et al. Crystal structure of S-ribosylhomocysteinase (LuxS) in complex with a catalytic 2-ketone intermediate[J]. Biochemistry, 2005, 44(10): 3745–3753. DOI: 10.1021/bi0477384 |
| [34] | HILGERS M T, LUDWIG M L. Crystal structure of the quorum-sensing protein LuxS reveals a catalytic metal site[J]. Proc Natl Acad Sci U S A, 2001, 98(20): 11169–11174. DOI: 10.1073/pnas.191223098 |
| [35] | RUZHEINIKOV S N, DAS S K, SEDELNIKOVA S E, et al. The 1.2 Å structure of a novel quorumsensing protein, Bacillus subtilis LuxS[J]. J Mol Biol, 2001, 313(1): 111–122. DOI: 10.1006/jmbi.2001.5027 |
| [36] | WANG Y, ZHANG W, WU Z F, et al. Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence[J]. Vet Microbiol, 2011, 152(1-2): 151–160. DOI: 10.1016/j.vetmic.2011.04.029 |
| [37] | WANG Y, YI L, WANG S H, et al. Crystal structure and identification of two key amino acids involved in AI-2 production and biofilm formation in Streptococcus suis LuxS[J]. PLoS One, 2015, 10(10): e0138826. DOI: 10.1371/journal.pone.0138826 |
| [38] | DING W, LI Y Z, ZHAO J F, et al. The catalytic mechanism of the class C radical S-adenosylmethionine methyltransferase NosN[J]. Angew Chem Int Ed Engl, 2017, 56(14): 3857–3861. DOI: 10.1002/anie.201609948 |
| [39] | KUNJAPUR A M, HYUN J C, PRATHER K L J. Deregulation of S-adenosylmethionine biosynthesis and regeneration improves methylation in the E. coli de novo vanillin biosynthesis pathway[J]. Microb Cell Fact, 2016, 15: 61. DOI: 10.1186/s12934-016-0459-x |
| [40] | PLUMMER P, ZHU J E, AKIBA M, et al. Identification of a key amino acid of LuxS involved in AI-2 production in Campylobacter jejuni[J]. PLoS One, 2011, 6(1): e15876. DOI: 10.1371/journal.pone.0015876 |
| [41] | BANERJEE G, RAY A K. The talking language in some major gram-negative bacteria[J]. Arch Microbiol, 2016, 198(6): 489–499. DOI: 10.1007/s00203-016-1220-x |
| [42] | WILLIAMS T C, AYRAPETYAN M, OLIVER J D. Molecular and physical factors that influence attachment of Vibrio vulnificus to chitin[J]. Appl Environ Microbiol, 2015, 81(18): 6158–6165. DOI: 10.1128/AEM.00753-15 |
| [43] | HAWVER L A, JUNG S A, NG W L. Specificity and complexity in bacterial quorum-sensing systems[J]. FEMS Microbiol Rev, 2016, 40(5): 738–752. DOI: 10.1093/femsre/fuw014 |
| [44] | SALINI R, PANDIAN S K. Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens[J]. Pathog Dis, 2015, 73(6): ftv038. |
| [45] | HAN X G, LU C P. Detection of autoinducer-2 and analysis of the profile of luxS and pfs transcription in Streptococcus suis serotype 2[J]. Curr Microbiol, 2009, 58(2): 146–152. DOI: 10.1007/s00284-008-9291-9 |
| [46] | WANG Y, YI L, ZHANG Z C, et al. Overexpression of luxS cannot increase autoinducer-2 production, only affect the growth and biofilm formation in Streptococcus suis[J]. Sci World J, 2013, 2013: 924276. |
| [47] | MA Y P, KE H, HAO L. LuxS/AI-2 quorum sensing is involved in antimicrobial susceptibility in Streptococcus agalactiae[J]. Fish Pathol, 2015, 50(1): 8–15. DOI: 10.3147/jsfp.50.8 |
| [48] |
杨登辉, 汪洋, 王少辉, 等. 密度感应系统luxS基因对鼠伤寒沙门菌生物学特性及毒力的影响[J]. 中国兽医科学, 2016, 46(5): 537–543.
YANG D H, WANG Y, WANG S H, et al. Effects of quorum sensing system luxS gene on the biological characteristics and virulence of Salmonella typhimurium[J]. Chinese Veterinary Science, 2016, 46(5): 537–543. (in Chinese) |
| [49] | WANG X, LI X L, LING J Q. Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans[J]. J Basic Microbiol, 2017, 57(7): 605–616. DOI: 10.1002/jobm.v57.7 |
| [50] |
汪洋. 猪链球菌生物被膜形成及致病机理研究[D]. 南京: 南京农业大学, 2011.
WANG Y. Study on the mechanism of biofilm formation and molecular pathogenesis of Streptococcus suis[D]. Nanjing: Nanjing Agricultural University, 2011. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10307-1012271118.htm |
| [51] | ALONSO B, CRUCES R, PÉREZ A, et al. Comparison of the XTT and resazurin assays for quantification of the metabolic activity of Staphylococcus aureus biofilm[J]. J Microbiol Methods, 2017, 139: 135–137. DOI: 10.1016/j.mimet.2017.06.004 |
| [52] | WANG Y, YI L, ZHANG Z C, et al. Biofilm formation, host-cell adherence, and virulence genes regulation of Streptococcus suis in response to autoinducer-2 signaling[J]. Curr Microbiol, 2014, 68(5): 575–580. DOI: 10.1007/s00284-013-0509-0 |
| [53] | REDMAN J A, WALKER S L, ELIMELECH M. Bacterial adhesion and transport in porous media:role of the secondary energy minimum[J]. Environ Sci Technol, 2004, 38(6): 1777–1785. DOI: 10.1021/es034887l |
| [54] | SEGURA M, FITTIPALDI N, CALZAS C, et al. Critical Streptococcus suis virulence factors:are they all really critical?[J]. Trends Microbiol, 2017, 25(7): 585–599. DOI: 10.1016/j.tim.2017.02.005 |
| [55] | FITTS E C, ANDERSSON J A, KIRTLEY M L, et al. New insights into autoinducer-2 signaling as a virulence regulator in a mouse model of pneumonic plague[J]. mSphere, 2016, 1(6): e00342–16. |
| [56] | SUGIURA T, OKUMIYA T, KUBO T, et al. Evaluation of intravascular hemolysis with erythrocyte creatine in patients with aortic stenosis[J]. Int Heart J, 2016, 57(4): 430–433. DOI: 10.1536/ihj.15-433 |
| [57] | ROLLAND J L, STIEN D, SANCHEZ-FERANDIN S, et al. Quorum sensing and quorum quenching in the phycosphere of phytoplankton:a case of chemical interactions in ecology[J]. J Chem Ecol, 2016, 42(12): 1201–1211. DOI: 10.1007/s10886-016-0791-y |
| [58] |
刘蕾, 桂萌, 武瑞赟, 等. LuxS/AI-2型群体感应系统调控细菌生物被膜形成研究进展[J]. 食品科学, 2016, 37(19): 254–262.
LIU L, GUI M, WU R Y, et al. Progress in research on biofilm formation regulated by LuxS/AI-2 quorum sensing[J]. Food Science, 2016, 37(19): 254–262. (in Chinese) |
| [59] | RYU E J, SIM J, SIM J, et al. D-galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens[J]. J Microbiol, 2016, 54(9): 632–637. DOI: 10.1007/s12275-016-6345-8 |
| [60] | RAO R M, PASHA S N, SOWDHAMINI R. Genome-wide survey and phylogeny of S-ribosylhomocysteinase (LuxS) enzyme in bacterial genomes[J]. BMC Genomics, 2016, 17(1): 742. DOI: 10.1186/s12864-016-3002-x |
| [61] | THOMPSON J A, OLIVEIRA R A, DJUKOVIC A, et al. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota[J]. Cell Rep, 2015, 10(11): 1861–1871. DOI: 10.1016/j.celrep.2015.02.049 |
| [62] | JOSHI J R, BURDMAN S, LIPSKY A, et al. Plant phenolic acids affect the virulence of Pectobacterium aroidearum and P. carotovorum ssp. brasiliense via quorum sensing regulation[J]. Mol Plant Pathol, 2016, 17(4): 487–500. DOI: 10.1111/mpp.12295 |
| [63] |
燕彩玲, 李博, 顾悦, 等. 信号分子AI-2的检测方法研究进展[J]. 微生物学通报, 2016, 43(6): 1333–1338.
YAN C L, LI B, GU Y, et al. Methods for the determination of autoinducer-2-a review[J]. Microbiology China, 2016, 43(6): 1333–1338. (in Chinese) |
| [64] |
汪洋, 易力, 张才, 等. 一种猪链球菌密度感应系统阻断多肽及其应用: 中国, CN102942617B[P]. 2014-05-14.
WANG Y, YI L, ZHANG C, et al. Streptococcus suis quorum sensing system blocking peptide and application of streptococcus suis quorum sensing system blocking peptide: China, CN102942617B[P]. 2014-05-14. (in Chinese) |
| [65] |
张勇, 王瑶, 陈士云. 群体感应信号分子AI-2研究进展[J]. 中国生物工程杂志, 2005, 25(9): 14–18.
ZHANG Y, WANG Y, CHEN S Y. Advances on quorum sensing AI-2 signal molecular[J]. China Biotechnology, 2005, 25(9): 14–18. (in Chinese) |



