2. 南京农业大学动物科技学院 动物遗传育种研究室, 南京 210095
2. Laboratory of Animal Genetics and Breeding, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
现代集约化动物生产过程中难以避免应激的发生[1-2]。应激是指动物机体对威胁自身稳态事件所产生的一系列生物反应。应激发生后,机体下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal,HPA)系统被激活,下丘脑释放促肾上腺皮质激素释放激素(corticotropin releasing hormone,CRH),CRH促使垂体释放促肾上腺皮质激素(adrenocorticotropic hormone,ACTH),ACTH作用于肾上腺皮质,上调皮质醇等肾上腺皮质类激素的分泌,从而调动机体各个组织器官应对外界刺激[3-4]。在猪生产中,应激被认为是损害猪生产性能的主要因素[5-6]。断奶或环境因素产生的应激会影响猪的采食量,降低猪日增重和饲料转化率,造成猪生长缓慢[7-8]。热应激会破坏猪的肠道屏障功能,降低部分消化酶活性,影响肠道微生物菌群平衡[9]。运输应激引起猪紧张不安,导致体温升高,降低猪肉品质[10]。除了对生长性能的影响,应激还会损害动物的免疫系统。研究发现,热应激后猪血清中细胞炎症因子IL-2、IFN-γ及TNF-α水平改变,机体的细胞免疫功能被抑制[11]。运输后猪的血清总蛋白下降,抗体生成减少,极易发生腹泻或呼吸道疾病甚至猝死[12-13]。对于生产母猪,温度升高会降低母猪的采食量及泌乳量,进而降低母猪体重,影响仔猪生长速率[14]。此外,应激会严重影响母猪的繁殖性能。应激导致母猪体内激素分泌紊乱,使母猪卵巢卵泡发育受阻、排卵减少,延迟母猪断奶后发情,严重时会诱发卵巢囊肿,增加母猪繁殖障碍的几率,给养殖场造成巨大的经济损失[15-17]。因此,研究应激反应的作用机制对了解猪的应激损伤意义重大。
血液中皮质醇浓度升高是衡量应激发生的重要指标。皮质醇刺激脂解和糖质新生,增加血糖供应,为机体应激应答提供更多的能量[18]。外源ACTH注射显著增加血液皮质醇水平,上调血糖水平,模拟应激的发生[19]。作为血糖来源的主要器官,肝中存在与糖原合成、糖原分解、糖酵解、糖异生、磷酸戊糖途径等糖代谢相关的酶类,它们的表达及活性是决定葡萄糖代谢正常进行的关键因素[20-22]。尽管近年来国内外对猪应激的研究取得了一定的进展,但应激对血糖指标变化的作用机理还有待进一步探讨。
为了明确应激引起的高皮质醇水平对母猪肝血糖调控机制的影响,本试验对母猪注射促肾上腺皮质激素(ACTH),通过检测母猪肝中糖代谢相关酶类的转录表达及酶活性,解析应激对肝血糖调控的影响机制,为进一步了解应激对母猪生产性能的影响提供一定的理论和试验依据。
1 材料与方法 1.1 试验动物及试验设计试验母猪由江苏淮阴种猪场提供。选取9头,体重为(185±25) kg的健康经产断奶苏淮母猪,产后40天断奶,随机分成处理组(5头)和对照组(4头)。断奶当天对母猪进行颈静脉插管手术,断奶后第2天开始试验(试验的第1天)。处理组于每天的8:00、16:00、24:00时(间隔8 h)分别注射1 IU·kg-1 ACTH,同时给对照组受试母猪注射等量生理盐水,连续注射7 d。试验母猪自由采食、饮水,于试验第8天进行屠宰取样。
1.2 试剂ACTH(A6303)购自Sigma公司;Trizol购自Invitrogen公司;M-MLV Reverse Transcriptase、SYBR Premix Ex Taq TM购自TaKaRa公司;皮质醇放射免疫分析试剂盒购自北京北方生物公司。葡萄糖代谢相关酶活性检测试剂盒购自索莱宝科技有限公司,具体货号:6-磷酸葡萄糖脱氢酶(BC0260)、丙酮酸激酶(BC0540)、己糖激酶(BC0740)、糖原磷酸化酶(BC3340)、糖原合成酶(BC3330)、丙酮酸羧化酶(BC0730)、磷酸烯醇式丙酮酸羧激酶(BC3310)、果糖-1, 6-二磷酸酶(BC0920)、葡萄糖-6-磷酸酶(BC3320)、果糖-6-磷酸激酶(BC0530)。
1.3 样品采集每天采用一次性负压采血管(含肝素钠)由颈静脉插管采血10 mL。所有血液样品经3 000 r·min-1离心10 min,分离血浆,于-20 ℃中保存,用于血糖和皮质醇水平的测定。试验母猪屠宰后,迅速采集肝组织,置于液氮中冻存。
1.4 血糖及皮质醇测定将分离的待测血浆送至南京市军区总医院,利用全自动生化分析仪对血糖(GLU)进行检测分析;采用放射免疫法测定血浆皮质醇浓度。
1.5 实时荧光定量PCR根据NCBI核酸数据库中相应基因mRNA的全序列,利用Primer premier5.0软件设计引物,引物由金唯智生物公司合成(引物序列见表 1)。Trizol法提取肝样品中的总RNA, 紫外分光光度计检测RNA质量和浓度,进行反转录及荧光定量PCR扩增。反应体系:2×SYBR Premix Ex Taq 10 μL,上下游引物(10 μmol·L-1)各0.4 μL,50×ROX Reference Dye 0.4 μL,cDNA模板2 μL,ddH2O 6.8 μL,总体积20 μL。反应条件:95 ℃预变性3 min;95 ℃变性10 s,62 ℃退火10 s,72 ℃延伸15 s,40个循环。被检样品均重复3次,取平均值;以GAPDH作为内参基因,利用2- △△Ct法计算各基因的相对表达量[23],△Ct = Ct目的基因-Ct内参基因;△△Ct=(Ct处理组目的基因-Ct处理组内参基因)-(Ct对照组目的基因-Ct对照组内参基因),2-△△Ct表示处理组目的基因的表达相对于对照组的变化倍数。
|
|
表 1 实时荧光定量PCR引物列 Table 1 Primers for the real-time quantitative PCR assay |
根据试剂盒说明书,取适量肝组织,按照组织质量(g):提取液体积(mL)为1:(5~10)的比例,冰浴匀浆;于8 000 g 4 ℃离心10 min,取上清,置冰上;分光光度计波长设置为340 nm,用蒸馏水调零,加样检测葡萄糖代谢相关酶活性。
1.7 数据统计分析所有数据均用“平均数±标准差”表示,利用SPSS 20.0软件进行统计,采用独立样本t检验分析试验组与对照组之间的差异;*表示处理组与对照组比较差异显著(P < 0.05),**表示差异极显著(P < 0.01)。
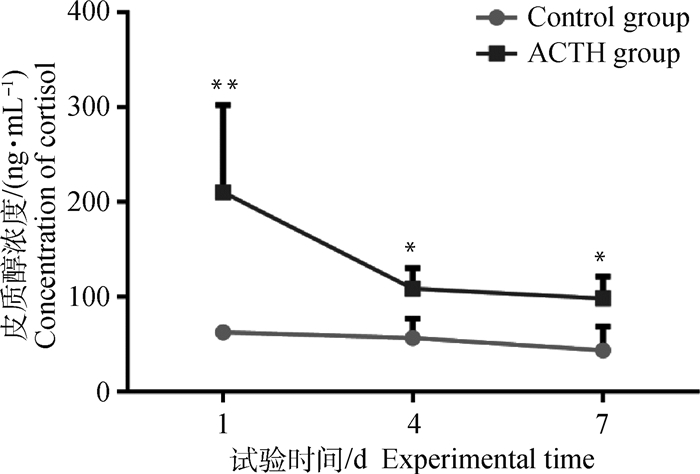
2 结果 2.1 ACTH处理对母猪血液皮质醇浓度及血糖水平的影响母猪血液皮质醇浓度变化如图 1所示。试验第1天,ACTH处理组母猪血液皮质醇浓度急剧升高,组间差异极显著(P < 0.01);试验第1到第4天,ACTH处理组皮质醇浓度下降,但仍显著高于对照组(P < 0.05);试验第4到第7天,处理组皮质醇浓度稳定,处理组与对照组差异显著(P < 0.05)。试验期间,对照组母猪血液中皮质醇浓度稳定,没有显著变化。
|
*表示处理组与对照组比较差异显著(P < 0.05),**表示差异极显著(P < 0.01)。下图同 * indicate significant difference (P < 0.05), ** indicate extremely significant difference (P < 0.01) between treatment and control groups. The same as the following figures 图 1 ACTH处理对母猪血液中皮质醇浓度的影响 Figure 1 Effects of ACTH-treatment on concentrations of cortisol in the blood of sows |
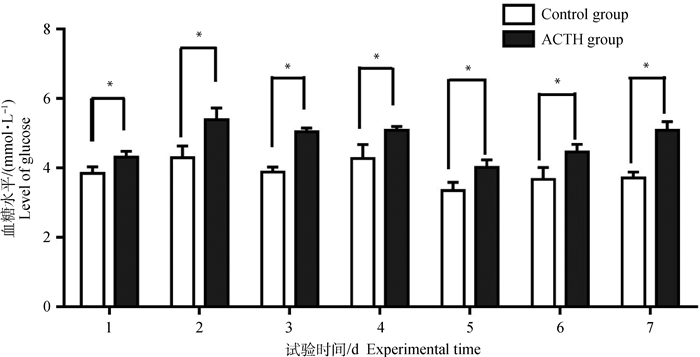
母猪血糖水平变化如图 2所示,试验期间处理组血糖水平均高于对照组,且同一时间段组间差异均达显著水平(P < 0.05)。
|
图 2 ACTH处理对母猪血糖水平的影响 Figure 2 Effects of ACTH-treatment on levels of glucose in the blood of sows |
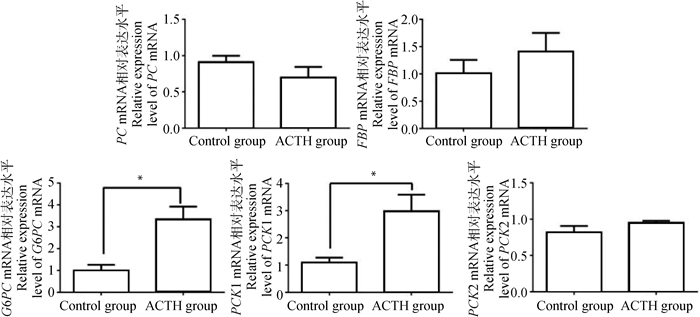
母猪肝糖异生相关酶类mRNA表达水平如图 3所示。对照组与处理组母猪肝中丙酮酸羧化酶(PC)、果糖-1, 6-二磷酸酶(FBP)及磷酸烯醇式丙酮酸羧激酶2(PCK2)的mRNA表达水平差异不显著(P > 0.05)。与对照组相比,ACTH处理组母猪肝中葡萄糖-6-磷酸酶(G6PC)及磷酸烯醇式丙酮酸羧激酶1(PCK1)的mRNA表达水平显著上调(P < 0.05)。
|
图 3 ACTH处理对母猪肝糖异生相关酶类mRNA表达的影响 Figure 3 Effects of ACTH-treatment on mRNA expression of enzymes related to gluconeogenesis in the liver of sows |
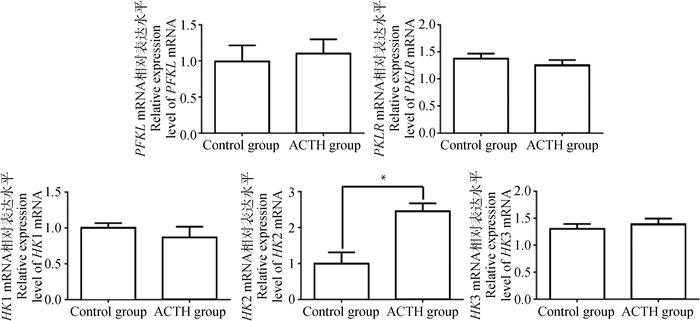
分析ACTH处理对母猪肝中糖酵解相关酶类mRNA表达的影响,结果如图 4所示。母猪肝中磷酸果糖激酶(PFKL)、丙酮酸激酶(PKLR)、己糖激酶1(HK1)和己糖激酶3(HK3)的mRNA表达水平在两组间差异不显著(P > 0.05)。但是,处理组母猪肝中己糖激酶2(HK2) mRNA表达水平显著高于对照组(P < 0.05)。
|
图 4 ACTH处理对母猪肝糖酵解相关酶类mRNA表达的影响 Figure 4 Effects of ACTH-treatment on mRNA expression of enzymes related to glycolysis in the liver of sows |
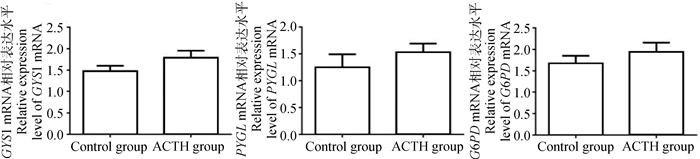
分析ACTH处理对母猪肝中糖原合成、分解及戊糖磷酸途径相关酶类mRNA表达的影响,结果如图 5所示。与对照组相比,处理组母猪肝中糖原合成酶1(GYS1)、糖原磷酸化酶(PYGL)及6-磷酸葡萄糖脱氢酶(G6PD)的mRNA表达水平呈上升趋势,但两组间差异均不显著(P > 0.05)。
|
图 5 ACTH处理对母猪肝糖原合成、分解及戊糖磷酸途径相关酶类mRNA表达的影响 Figure 5 Effects of ACTH-treatment on mRNA expression of enzymes related to glycogen synthesis, breakdown and pentose phosphate pathway in the liver of sows |
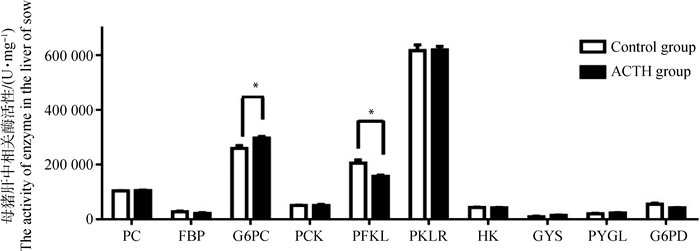
ACTH处理对母猪肝中葡萄糖代谢相关酶类活性的影响如图 6所示。处理组母猪肝中丙酮酸羧化酶(PC)、果糖-1, 6-二磷酸酶(FBP)、磷酸烯醇式丙酮酸羧激酶(PCK)的活性与对照组相比差异不显著(P > 0.05)。处理组母猪肝中葡萄糖-6-磷酸酶(G6PC)的活性显著高于对照组(P < 0.05),磷酸果糖激酶(PFKL)的活性显著低于对照组(P < 0.05)。此外,丙酮酸激酶(PKLR)、己糖激酶(HK)、糖原合成酶(GYS)、糖原磷酸化酶(PYGL)及6-磷酸葡萄糖脱氢酶(G6PD)活性在两组间的差异均不显著(P > 0.05)。
|
图 6 ACTH处理对母猪肝中葡萄糖代谢相关酶活性的影响 Figure 6 Effects of ACTH-treatment on activity of enzymes related to glucose metabolism in the liver of sows |
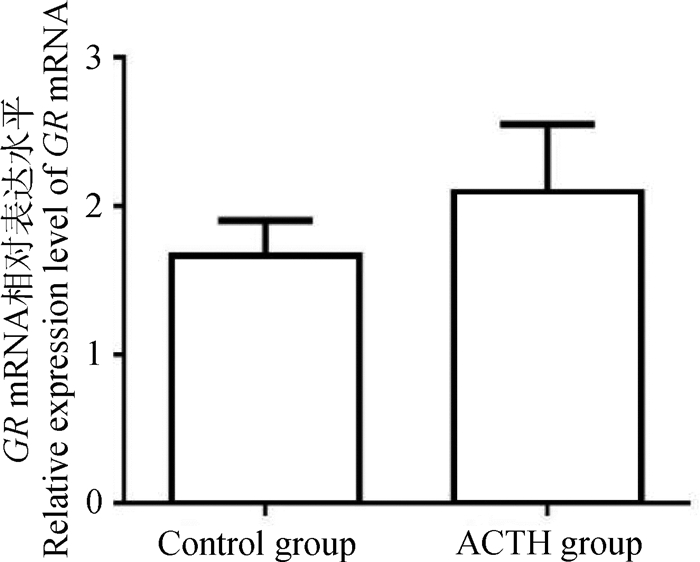
检测处理组与对照组母猪肝中糖皮质激素受体(GR) mRNA的表达水平,结果显示, 处理组GR水平呈上调趋势,但两组间差异不显著(P > 0.05)(图 7)。
|
图 7 ACTH处理对母猪肝中糖皮质激素受体mRNA表达的影响 Figure 7 Effects of ACTH-treatment on mRNA expression of glucocorticoid receptor in the liver of sows |
本试验通过对母猪注射促肾上腺皮质激素(ACTH)模拟应激发生,分析应激对母猪肝血糖调控的影响机制。试验第1天,处理组皮质醇浓度急剧升高,随着试验持续进行,皮质醇浓度略有下降,这可能与皮质醇对HPA轴的反馈调节有关。对照组皮质醇浓度保持平稳,说明注射处理本身没有对动物造成应激反应。试验期间处理组母猪皮质醇浓度一直显著高于对照组,且处理组血糖水平明显高于对照组,均反映处理组母猪处于应激状态,应激模型建立成功。
血糖是体内代谢过程中的重要物质,与部分血脂指标及脂肪沉积性状存在显著相关性[24],可以反映机体能量变化。肝糖异生是肝糖代谢的重要组成部分,机体近一半葡萄糖的供应依赖于糖异生作用[25]。糖异生的速度与编码糖异生途径限速酶的基因表达有关[26-27]。本试验中,处理组肝母猪肝中糖异生途径的关键酶葡萄糖-6-磷酸酶(G6PC)及磷酸烯醇式丙酮酸羧激酶(PCK1、PCK2)的mRNA表达水平均上调,且葡萄糖-6-磷酸酶(G6PC)的酶活性明显上升,表明ACTH处理引起的高浓度皮质醇可能通过糖异生途径中的限速酶葡萄糖-6-磷酸酶及磷酸烯醇式丙酮酸羧激酶加速肝中糖异生速度,提高血液中葡萄糖浓度。糖酵解是机体内分解葡萄糖供能的必经途径,酵解过程有3步反应为不可逆反应,催化这3步反应的酶是己糖激酶、磷酸果糖激酶和丙酮酸激酶,其中磷酸果糖激酶是糖酵解反应的限速酶[28-30]。本试验中,处理组肝己糖激酶2(HK2)mRNA表达上调;己糖激酶1(HK1)、丙酮酸激酶(PKLR)的mRNA表达水平呈下调趋势。在正常生理状态下,低浓度血糖会激起肝中丙酮酸激酶的磷酸化,使其活性降低,降低肝中葡萄糖的利用[31]。本研究中,ACTH处理下调丙酮酸激酶的转录表达,降低磷酸果糖激酶的酶活性,暗示应激抑制猪肝中的糖酵解,进而抑制肝中葡萄糖的利用。糖原是葡萄糖的一种高效能的贮存形式,糖原的存在保证了机体最需能量供应的脑和肌肉紧张活动时对能量的需要,同时也保证不间断地供给恒定水平的血糖[32-33]。肝是机体贮存糖原的器官,本研究中,ACTH处理没有显著影响肝中糖原合成酶1(GYS1)的mRNA表达,此外参与糖原分解的糖原磷酸化酶(PYGL)及参与磷酸戊糖途径的关键酶6-磷酸葡萄糖脱氢酶(G6PD)的mRNA表达均未发生显著变化,暗示ACTH处理未影响肝中糖原合成、糖原分解及磷酸戊糖途径。
皮质醇主要通过与糖皮质激素受体结合发挥其生物学作用[34-35],为分析ACTH处理是否影响母猪肝中糖皮质激素受体GR的表达,本研究分析了两组母猪肝中糖皮质激素受体mRNA的表达差异,结果显示,处理组猪肝中糖皮质激素受体水平呈上调趋势,但差异未达显著水平;这一结果暗示,ACTH处理对母猪肝中糖代谢相关酶类转录活性的影响可能依赖于GR之外的其它途径,具体机制仍需要进一步研究探讨。
4 结论本试验通过ACTH处理模拟猪生产中应激的发生,致使母猪皮质醇浓度升高,其导致血糖水平升高的可能作用机制:一方面通过上调肝中磷酸烯醇式丙酮酸羧激酶和葡萄糖-6-磷酸酶基因的转录表达,增强糖异生作用;另一方面,通过下调肝中磷酸果糖激酶的活性,抑制糖酵解反应,降低葡萄糖利用。但ACTH处理不影响肝中磷酸戊糖途径、糖原合成及分解;且ACTH处理未影响母猪肝中糖皮质激素受体的转录表达,提示ACTH处理对母猪肝中糖代谢相关酶类转录活性的影响可能依赖于GR之外的其它途径。
| [1] | CANARIO L, MIGNON-GRASTEAU S, DUPONT-NIVET M, et al. Genetics of behavioural adaptation of livestock to farming conditions[J]. Animal, 2013, 7(3): 357–377. DOI: 10.1017/S1751731112001978 |
| [2] | KOOLHAAS J M, VAN REENEN C G. Animal behavior and well-being symposium:interaction between coping style/personality, stress, and welfare:relevance for domestic farm animals[J]. J Anim Sci, 2016, 94(6): 2284–2296. DOI: 10.2527/jas.2015-0125 |
| [3] | TOUFEXIS D, RIVAROLA M A, LARA H, et al. Stress and the reproductive axis[J]. J Neuroendocrinol, 2014, 26(9): 573–586. DOI: 10.1111/jne.2014.26.issue-9 |
| [4] | LOPEZ-DURAN N L, MAYER S E, ABELSON J L. Modeling neuroendocrine stress reactivity in salivary cortisol:adjusting for peak latency variability[J]. Stress, 2014, 17(4): 285–295. DOI: 10.3109/10253890.2014.915517 |
| [5] |
李玮, 刘蕤, 马尧, 等. 重度冷应激对三河牛血液生化指标及相关基因表达的影响[J]. 畜牧兽医学报, 2015, 46(8): 1463–1470.
LI W, LIU R, MA Y, et al. Effects of severe cold stress on blood biochemical parameters and related gene expression in Sanhe cattle[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(8): 1463–1470. (in Chinese) |
| [6] | RUTHERFORD K M D, PIASTOWSKA-CIESIELSKA A, DONALD R D, et al. Prenatal stress produces anxiety prone female offspring and impaired maternal behaviour in the domestic pig[J]. Physiol Behav, 2014, 129: 255–264. DOI: 10.1016/j.physbeh.2014.02.052 |
| [7] | CARROLL J A, BURDICK N C, CHASE C C Jr, et al. Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to a provocative immune challenge[J]. Domest Anim Endocrinol, 2012, 43(2): 146–153. DOI: 10.1016/j.domaniend.2011.12.008 |
| [8] |
邹晓庭, 胡家澄, 曹德瑞, 等. γ-氨基丁酸对夏季高温期生长肥育猪生产性能、抗氧化及HPA、HPT轴激素分泌的影响[J]. 畜牧兽医学报, 2009, 40(8): 1196–1201.
ZOU X T, HU J C, CAO D R, et al. Effect of γ-aminobutyric acid on growth performance, antioxidation and hormones of HPA, HPT axis in growth-finishing swine in hot summer[J]. Acta Veterinaria et Zootechnica Sinica, 2009, 40(8): 1196–1201. DOI: 10.3321/j.issn:0366-6964.2009.08.011 (in Chinese) |
| [9] |
熊云霞, 马现永, 郑春田, 等. 热应激对猪肠道健康、免疫系统和肉品质的影响及作用机制[J]. 动物营养学报, 2017, 29(2): 374–381.
XIONG Y X, MA X Y, ZHENG C T, et al. Effects of heat stress on intestinal health, immune system and meat quality in pigs and its mechanisms[J]. Chinese Journal of Animal Nutrition, 2017, 29(2): 374–381. DOI: 10.3969/j.issn.1006-267x.2017.02.002 (in Chinese) |
| [10] |
柴进, 彭健, 熊琪, 等. 宰前休息方式对猪福利、血液成分及肉质的影响[J]. 畜牧兽医学报, 2009, 40(11): 1645–1650.
CHAI J, PENG J, XIONG Q, et al. The influence of lairage conditions for finishing pigs on mental performance, blood index and meat quality[J]. Acta Veterinaria et Zootechnica Sinica, 2009, 40(11): 1645–1650. DOI: 10.3321/j.issn:0366-6964.2009.11.011 (in Chinese) |
| [11] |
胡艳欣, 佘锐萍, 张洪玉, 等. 热应激后猪血清中IL-2、IFN-γ及TNF-α水平的动态变化[J]. 畜牧兽医学报, 2006, 37(5): 496–499.
HU Y X, SHE R P, ZHANG H Y, et al. Studies on the dynamic changes of the level of il-2, ifn-γ and tnf-α in porcine serum after heat stress[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(5): 496–499. DOI: 10.3321/j.issn:0366-6964.2006.05.015 (in Chinese) |
| [12] |
高得仪, 韩博, 王清兰, 等. 猪运输应激血液生化指标变化[J]. 中国兽医学报, 1996, 16(3): 285–289.
GAO D Y, HAN B, WANG Q L, et al. The alterations of blood biochemical indexes of pig due to transportation stress[J]. Chinese Journal of Veterinary Science, 1996, 16(3): 285–289. (in Chinese) |
| [13] |
吕琼霞, 张书霞, 赵茹茜. 运输应激对猪免疫机能的影响及其细胞因子的调控[J]. 中国农业科学, 2009, 42(7): 2579–2585.
LV Q X, ZHANG S X, ZHAO R Q. Effects of transportation stress on immune function and regulation of cytokines in pigs[J]. Scientia Agricultura Sinica, 2009, 42(7): 2579–2585. (in Chinese) |
| [14] | KELLNER T A, BAUMGARD L H, PRUSA K J, et al. Does heat stress alter the pig's response to dietary fat?[J]. J Anim Sci, 2016, 94(11): 4688–4703. DOI: 10.2527/jas.2016-0756 |
| [15] | RAULT J L, MORRISON R S, HANSEN C F, et al. Effects of group housing after weaning on sow welfare and sexual behavior[J]. J Anim Sci, 2014, 92(12): 5683–5692. DOI: 10.2527/jas.2014-8238 |
| [16] | POLING M C, LUO E Y, KAUFFMAN A S. Sex differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and rfrp-3 neurons may contribute to the sexually dimorphic basis of the LH surge[J]. Endocrinology, 2017, 158(10): 3565–3578. DOI: 10.1210/en.2017-00405 |
| [17] | WILLIAMS A M, SAFRANSKI T J, SPIERS D E, et al. Effects of a controlled heat stress during late gestation, lactation, and after weaning on thermoregulation, metabolism, and reproduction of primiparous sows[J]. J Anim Sci, 2013, 91(6): 2700–2714. DOI: 10.2527/jas.2012-6055 |
| [18] | TERENINA E, SAUTRON V, YDIER C, et al. Time course study of the response to LPS targeting the pig immune gene networks[J]. BMC Genomics, 2017, 18: 988. DOI: 10.1186/s12864-017-4363-5 |
| [19] | BELDA X, FUENTES S, DAVIU N, et al. Stress-induced sensitization:the hypothalamic- pituitary-adrenal axis and beyond[J]. Stress, 2015, 18(3): 269–279. DOI: 10.3109/10253890.2015.1067678 |
| [20] | WATANABE H, INABA Y, KIMURA K, et al. Sirt2 facilitates hepatic glucose uptake by deacetylating glucokinase regulatory protein[J]. Nat Commun, 2018, 9(1): 30. DOI: 10.1038/s41467-017-02537-6 |
| [21] | BEZBORODKINA N N, CHESTNOVA A Y, OKOVITY S V, et al. Activity of glycogen synthase and glycogen phosphorylase in normal and cirrhotic rat liver during glycogen synthesis from glucose or fructose[J]. Exp Toxicol Pathol, 2014, 66(2-3): 147–154. DOI: 10.1016/j.etp.2013.12.001 |
| [22] | JONES J G. Hepatic glucose and lipid metabolism[J]. Diabetologia, 2016, 59(6): 1098–1103. DOI: 10.1007/s00125-016-3940-5 |
| [23] | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the method[J]. Methods, 2001, 25(4): 402–408. DOI: 10.1006/meth.2001.1262 |
| [24] |
季久秀, 方绍明, 张震, 等. 猪血糖、血脂与脂肪沉积性状的关联性分析[J]. 畜牧兽医学报, 2016, 47(4): 686–692.
JI J X, FANG S M, ZHANG Z, et al. Correlations of blood glucose and lipids with fat deposition in pigs[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(4): 686–692. (in Chinese) |
| [25] | AZZOUT-MARNICHE D, GAUDICHON C, BLOUET C, et al. Liver glyconeogenesis:a pathway to cope with postprandial amino acid excess in high-protein fed rats?[J]. Am J Physiol Regul Integr Comp Physiol, 2007, 292(4): R1400–R1407. DOI: 10.1152/ajpregu.00566.2006 |
| [26] | GIMÉNEZ-CASSINA A, GARCIA-HARO L, CHOI C S, et al. Regulation of hepatic energy metabolism and gluconeogenesis by BAD[J]. Cell Metab, 2014, 19(2): 272–284. DOI: 10.1016/j.cmet.2013.12.001 |
| [27] | CONTI R, MANNUCCI E, PESSOTTO P, et al. Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis[J]. Diabetes, 2011, 60(2): 644–651. DOI: 10.2337/db10-0346 |
| [28] | MAREK C B, PERALTA R M, ITINOSE A M, et al. Influence of tamoxifen on gluconeogenesis and glycolysis in the perfused rat liver[J]. Chem-Biol Interact, 2011, 193(1): 22–33. DOI: 10.1016/j.cbi.2011.04.010 |
| [29] | RUI L Y. Energy metabolism in the liver[J]. Compr Physiol, 2014, 4(1): 177–197. |
| [30] | GREMPLER R, ZIBROVA D, SCHOELCH C, et al. Normalization of prandial blood glucose and improvement of glucose tolerance by liver-specific inhibition of SH2 domain-containing inositol phosphatase 2 (SHIP2) in diabetic KKAy mice:SHIP2 inhibition causes insulin-mimetic effects on glycogen metabolism, gluconeogenesis, and glycolysis[J]. Diabetes, 2007, 56(9): 2235–2241. DOI: 10.2337/db06-1660 |
| [31] | KAWAGUCHI T, TAKENOSHITA M, KABASHIMA T, et al. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein[J]. Proc Natl Acad Sci U S A, 2001, 98(24): 13710–13715. DOI: 10.1073/pnas.231370798 |
| [32] | GALLIS J L, GIN H, ROUMES H, et al. A metabolic link between mitochondrial ATP synthesis and liver glycogen metabolism:NMR study in rats re-fed with butyrate and/or glucose[J]. Nutr Metab (Lond), 2011, 8(1): 38. DOI: 10.1186/1743-7075-8-38 |
| [33] | LÓPEZ-SOLDADO I, FUENTES-ROMERO R, DURAN J, et al. Effects of hepatic glycogen on food intake and glucose homeostasis are mediated by the vagus nerve in mice[J]. Diabetologia, 2017, 60(6): 1076–1083. DOI: 10.1007/s00125-017-4240-4 |
| [34] | TELES M, BOLTAÑA S, REYES-LÓPEZ F, et al. Effects of chronic cortisol administration on global expression of GR and the liver transcriptome in Sparus aurata[J]. Mar Biotechnol (NY), 2013, 15(1): 104–114. DOI: 10.1007/s10126-012-9467-y |
| [35] | GUO C M, WANG W S, LIU C, et al. Induction of PGF2α synthesis by cortisol through GR dependent induction of CBR1 in human amnion fibroblasts[J]. Endocrinology, 2014, 155(8): 3017–3024. DOI: 10.1210/en.2013-1848 |



