2. 四川农业大学 动物疫病与人类健康四川省重点实验室, 成都 611130;
3. 四川农业大学 动物医学院禽病防治研究中心, 成都 611130
2. Sichuan Key Laboratory of Animal Diseases and Human Health, Sichuan Agricultural University, Chengdu 611130, China;
3. College of Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130, China
鸭甲肝病毒(duck hepatitis A virus,DHAV)是引起鸭病毒性肝炎(duck viral hepatitis)的病原之一,主要感染4周以内雏鸭,死亡雏鸭表现为肝肿胀、出血等特点。目前,DHAV划分为3个基因型[1]:DHAV-1在世界范围内分布,DHAV-2主要发生于我国台湾地区[2],DHAV-3分布在我国大陆地区[3-5]、韩国[6-7]和越南[8]。近年来,DHAV-3的流行呈现扩展趋势[8-15],给鸭养殖业带来巨大威胁。
DHAV-3感染发病雏鸭很难通过临床症状和剖检变化与DHAV-1感染发病雏鸭进行区分,但可通过血清学和分子生物学方法进行鉴别诊断。目前已建立多种可鉴别DHAV-1和DHAV-3的RT/rRT-PCR和免疫组化的方法并广泛应用到DHAV增殖规律的研究中[15-19]。DHAV在雏鸭体内增殖除由病毒自身特性决定外,还受初始感染剂量[18]、感染途径[20]、雏鸭日龄[21]、雏鸭种系[22]等多种因素影响。DHAV在雏鸭体内具有广泛的组织嗜性,但各组织病毒含量不同。普遍认为肝是DHAV增殖的最佳靶器官,其致病机制尚不清楚。张焕荣等[16]认为DHAV-1弱毒进入雏鸭肝后,可诱导多种细胞因子表达量上调,而其中干扰素(主要是IFN-α)转录水平的升高抑制了病毒增殖。C. Q. Gu等[23]研究表明DHAV-1 JX株感染24 h后病毒维持高水平,肝中细胞因子(TNF-α、IFN-α、IL-6、IL-10)表达量受到明显抑制。而C. P. Song等[24]检测结果表明DHAV-1强毒和弱毒均能诱导肝中多种细胞因子表达量上调,但上调程度不同,强毒诱导IFN-γ和IL-2的表达量上调更明显。目前尚未见DHAV-3在肝中致病机制的研究报道。本研究建立DHAV-3强毒人工感染7日龄雏鸭模型,并对病毒在雏鸭体内动态分布及诱导肝组织病变和细胞因子(IFN-α/β/γ和IL-1β/2/6)表达的关联性进行分析,以期为DHAV-3致病机制的研究奠定基础。
1 材料与方法 1.1 毒株和实验动物DHAV-3 QL株,由四川农业大学禽病中心分离鉴定并保存,对4日龄雏鸭的LD50=10-4.13·0.2 mL-1;1日龄北京鸭,购自四川农业大学家禽养殖场,隔离饲养至7日龄,参照Q. Hu等[5]和S. Mao等[25]方法检测DHAV及母源抗体含量均为阴性。
1.2 主要仪器和试剂CFX ConnectTMOptics Module Real-Time PCR System(Bio-Rad);RNAiso Plus (No.: 9109)、One Step PrimeScriptTMRT-PCR Kit(No.: RR064A)、PrimeScriptTM RT Kit(No.:RR047)购自TaKaRa;DHAV-3型引物和探针[5]由上海基康生物技术有限公司合成;6种细胞因子(IFN-α、β、γ和IL-1β、2、6)及内参GAPDH基因引物[26]由英潍捷基公司合成。
1.3 DHAV-3人工感染7日龄雏鸭疾病模型构建及样品采集77只7日龄雏鸭随机分为试验组(50只)和对照组(27只),试验组雏鸭腿部肌肉注射DHAV-3病毒6×107.99 copies·只-1(2 000 LD50),对照组腿部肌肉注射生理盐水(0.75%)0.5 mL·只-1。接毒后观察雏鸭临床发病和死亡情况,并于感染后1、3、6、9、12、24、48、72和96 h,在试验组(出现临床症状后,选择发病症状典型的雏鸭)和对照组中各采集3只雏鸭的血液、肝、脾、肺、肾、胰、法氏囊、胸腺、大脑、哈德氏腺和十二指肠,死亡雏鸭采集肝,置于-80 ℃保存备用,未死亡雏鸭取部分肝组织固定于4%多聚甲醛中备用。
1.4 定量检测DHAV-3含量及数据分析根据RNAiso Plus操作说明书,提取样品中总RNA,利用一步法TaqMan探针实时荧光定量RT-PCR方法[5]检测DHAV-3含量。检测结果用Graphpad prism 6中独立样本t检验方法分析并作图。
1.5 肝组织病理学观察及细胞因子检测对“1.3”保存的肝组织样品切片进行HE染色,光学显微镜观察,用Adobe photoshop CS 6作图对比。参照2-ΔΔCt相对定量方法[27]在转录水平检测肝中抗病毒细胞因子IFN-α、IFN-β和IFN-γ及促炎因子IL-1β、IL-2和IL-6,检测结果用Graphpad prism 6分析并作图。
2 结果 2.1 DHAV-3感染雏鸭的临床发病症状、病理变化及死亡情况雏鸭于感染18 h后开始出现症状,大部分雏鸭精神沉郁、扎堆,采食、饮水量明显减少;感染24 h后开始出现死亡,24 h死亡6只雏鸭,30 h死亡2只,48 h死亡2只,54 h死亡1只。剖检发病死亡雏鸭,可见肝肿大,表面分布有大量出血点或出血斑,质地变脆;脾充血呈钝圆形;肾有明显出血现象;胸腺也有出血点。对照组雏鸭精神、采食和饮水正常,剖检未发现病变。
用于单独观测DHAV-3致死率的雏鸭,共死亡4只(死亡率40%),24 h死亡2只,38 h死亡1只,60 h死亡1只。雏鸭生存曲线如图 1。
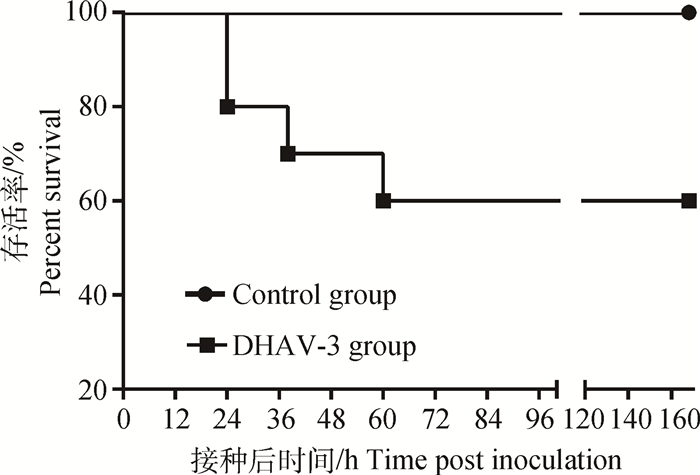
|
图 1 致病性DHAV-3感染7日龄雏鸭的生存曲线 Figure 1 Survival curves of 7-day-old ducklings after infected with virulent strain of DHAV-3 |
DHAV-3感染7日龄雏鸭后,1 h即在所有组织器官中检测到。但在各组织器官中含量不同,肝病毒含量最高(为106.34 copies·g-1),其次是脾、肾和血液中病毒含量,分别为105.56、105.03和105.04 copies·mL-1,其余器官病毒含量均在105 copies·g-1以下(图 2)。随时间推移,所有组织和器官中病毒含量均逐渐上升,血液和胰分别于感染后12和96 h含量最高,其余组织均于24~48 h达到峰值,随后病毒含量下降。其中,肝、脾和肾中各时间点DHAV-3含量明显高于其他组织器官,感染后24 h达到峰值,分别为1011.15、1010.37和1010.30 copies·g-1,48~96 h病毒含量缓慢下降(图 2A)。DHAV-3在除脾外其他免疫器官(胸腺、法氏囊、哈德氏腺)中增殖规律存在差异,胸腺中各时间点病毒含量高于法氏囊和哈德氏腺,而法氏囊峰值时间在感染后24 h早于胸腺和哈德氏腺峰值时间48 h(图 2B)。DHAV-3在血液组织和胰中增殖规律与其他器官不同,感染12 h后血液中病毒含量即达到峰值,为108.80 copies·mL-1,12~96 h病毒含量下降,96 h下降至105.69 copies·mL-1。DHAV-3在胰中含量持续增加,感染96 h后最高,为108.33 copies·g-1(图 2D)。DHAV-3在其他器官(肺、大脑、十二指肠)中增殖均在感染后24 h达到峰值,除9和12 h外各时间点病毒含量,十二指肠高于肺和大脑。大脑中病毒含量最低,峰值含量仅为107.66 copies·g-1,感染96 h后下降至105.60 copies·g-1(图 2C)。
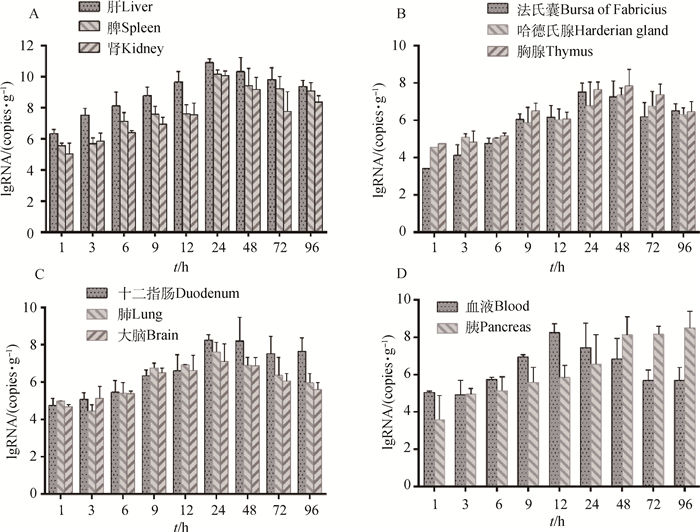
|
A.DHAV-3在肝、脾和肾增殖;B. DHAV-3在胸腺、法氏囊和哈德氏腺中增殖;C. DHAV-3在肺、大脑和十二指肠中增殖;D. DHAV-3在血液和胰中增殖 A. DHAV-3 proliferates in the liver, spleen and kidneys; B. DHAV-3 proliferates in thymus, bursa of Fabricius and Harderian gland; C. DHAV-3 proliferates in the lung, brain and duodenum; D. DHAV-3 proliferates in blood and pancreas 图 2 DHAV-3在各组织器官中动态分布 Figure 2 Distribution of DHAV-3 in different tissues and organs |
比较相邻时间点肝中DHAV-3病毒含量,分析其含量变化及显著性差异,以研究病毒的增殖速度。结果表明,感染1~3 h肝中病毒增殖速度最快,3 h病毒含量较1 h时有极显著差异(P < 0.01),而在9~12 h和12~24 h有显著性差异(P < 0.05)(图 3)。感染死亡雏鸭的肝病毒含量最高为1012.01 copies·g-1,最低1011.05 copies·g-1,平均含量为1011.47±0.37 copies·g-1。
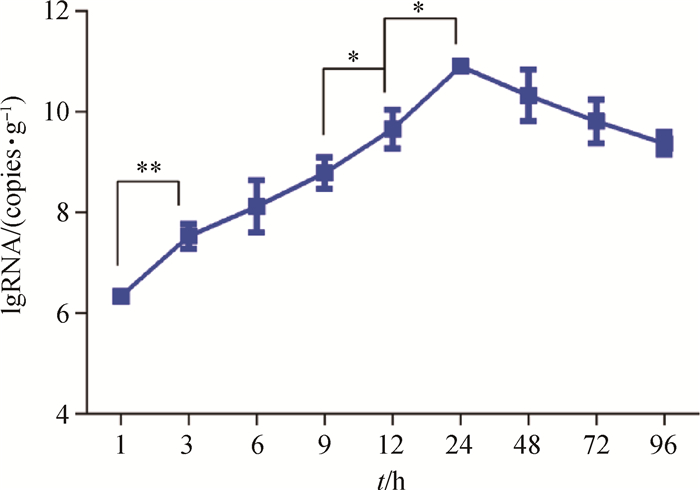
|
*. P < 0.05; **. P < 0.01 图 3 感染后不同时间点肝中DHAV-3载量 Figure 3 Viral load of DHAV-3 in the liver at different time points after infection |
通过光学显微镜观察DHAV-3感染雏鸭各时间点的肝病理组织学变化,结果显示:感染1 h,局部肝组织毛细血管充血,肝细胞轻微肿胀(图 4A,箭头所示);感染3 h,肝细胞呈空泡变性,细胞质内出现大小不一的空泡(图 4b,箭头所示);感染6 h,肝细胞肿胀明显,空泡变性加重,变性的肝细胞数目增多(图 4c,箭头所示);感染9 h,肝细胞呈严重空泡变性,肝组织呈网状,细胞核悬于中央或被挤于一侧(图 4 D 和d,箭头所示);感染12 h,中央静脉周围肝细胞仍以空泡变性为主,并伴有少量炎性细胞浸润(图 4e,箭头所示);感染24~48 h,肝中央静脉及汇管区周围肝细胞大多呈坏死性变化,细胞核固缩消失,细胞质碎裂呈碎片状或消失形成空泡,周围伴有大量易嗜性粒细胞浸润(图 4f、g,箭头所示);感染72~96 h,汇管区胆管上皮细胞呈明显增生性变化,周围肝细胞多呈空泡性变化并伴有少量黄染的枯否细胞。对照组未观察到病变。
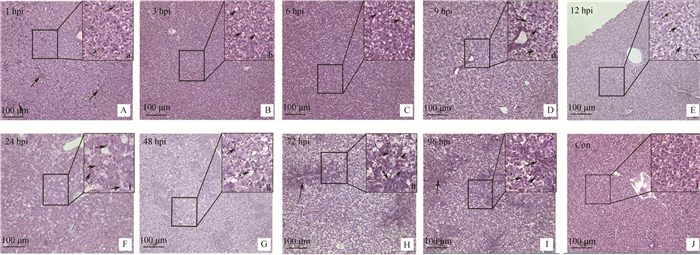
|
A~J.光学显微镜观察,200X;a~:j.光学显微镜观察,600X;A.感染1 h,肝毛细血管充血(箭头指示);B.感染3 h,肝细胞的细胞质内出现空泡(b,箭头指示);C.感染6 h,大量肝细胞内形成空泡(c,箭头指示);D.感染9 h,肝组织透明呈网状,细胞核悬于中央(d,箭头指示);E.感染12 h,肝细胞呈空泡变性,同时伴有炎性细胞浸润(e,箭头指示);F.感染24 h,肝细胞核固缩消失,细胞质碎裂形成空泡,少量易嗜性粒细胞浸润(f,箭头所指);G.感染48 h,肝细胞的细胞质消失形成空泡,大量易嗜性粒细胞浸润(g,箭头指示);H.感染72 h,汇管区胆管上皮细胞增生,伴有少量枯否细胞(H、h,箭头指示);I.感染96 h汇管区周围增生,少量炎性细胞浸润(I、i,箭头指示);J.空白对照组 A-J. Observed by optical microscope, 200 X; a-j. Observed by optical microscope, 600 X; A. Congestion in liver capillaries (arrow instructions) at 1 h PI; B. Emergence of vacuoles in the liver cells (b, arrow instructions) at 3 h PI; C. The vacuoles in the liver cells become larger (c, arrow instructions) at 6 h PI; D. Liver cells were transparent reticular, nucleus hanging in the central (d, arrows instructions) at 9 h PI; E. Lymphocyte infiltration (e, arrows instructions) at 12 PI; F. Hepatocyte cytoplasmic cleavage, a few heterophils distributed (f, arrows instructions) at 24 h PI; G. Hepatocyte lysis, heterophils infiltration (g, arrows instructions) at 48 h PI; H. Bile duct hyperplasia in the portal area, a small amount of Kupffer's cells (H, h, arrows instructions) were distributed between the liver cells at 72 h PI; I. The nucleus is condensed, gathered around the portal area, lymphocyte infiltration (I, i, arrows instructions) at 96 h PI; J. Blank control group 图 4 感染雏鸭肝组织的病理变化 Figure 4 Histopathological changes of liver in DHAV-3 infected ducklings |
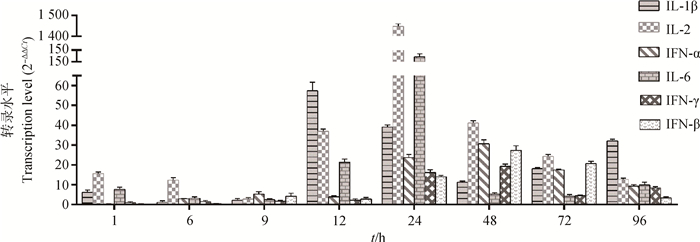
染料法实时荧光定量RT-PCR检测肝中干扰素及促炎性因子IL-1β、IL-2和IL-6各时间点含量变化,结果显示,抗病毒细胞因子(IFN-α、IFN-β、IFN-γ)的DHAV-3感染后表达量逐渐升高,在感染48 h后达到峰值,上调倍数分别为30.60、27.31和19.15倍,72~96 h上调倍数下降。促炎因子(IL-1β、IL-2、IL-6)在感染前期(1~9 h)变化不明显,感染12 h后显著增高,24 h后达峰值,感染48~96 h逐渐下降。其中IL-1β峰值出现在感染后12 h,早于IL-2和IL-6(24 h),但IL-2表达量上调最高,达1 448.25倍,高于IL-6和IL-1β的上调倍数分别为132.51和59.3倍(图 5)。
|
图 5 感染雏鸭肝细胞因子mRNA转录水平 Figure 5 Transcription level of cytokine mRNAs in liver of infected ducklings |
生产实践中,往往使用疫苗免疫种鸭,通过母源抗体而使雏鸭获得被动的保护,但母源抗体在7日龄后常常下降到较低水平[28-29],而使7日龄雏鸭感染DHAV后发病。雏鸭日龄对DHAV的致病性具有重要影响[23, 30-31],如苏敬良等[3]报道DHAV-3(G株)感染2日龄雏鸭的死亡率为80%,感染7日龄雏鸭的死亡率为40%,而感染13日龄的雏鸭则不致死;本研究结果相似。DHAV-3 (QL株)在4日龄雏鸭上测得LD50=10-4.13·0.2 mL-1,而以2 000 LD50剂量的DHAV-3强毒感染7日龄雏鸭的致死率为40%(图 1)。目前针对DHAV-3的研究所建立的人工感染雏鸭病理模型很多,但主要集中在4日龄以内[15-19],因此,本研究建立DHAV-3人工感染7日龄雏鸭的疾病模型,测定病毒在其体内分布规律、对肝组织病理学损伤、肝细胞因子含量变化等,分析病毒分布与病理损伤、抗病毒细胞因子、促炎性因子的关联性,对进一步阐明DHAV-3的致病机制具有重要意义。
3.2 DHAV-3在雏鸭体内的分布规律荧光定量结果显示,DHAV-3在不同组织中病毒含量不同。DHAV-3感染1 h即可在所有组织器官中检测到。这与林少莉等[18]和Q. X. Huang等[15]检测结果相似,表明DHAV-3有广泛的组织嗜性。但本研究中病毒全身性分布的时间要早。原因可能是攻毒剂量、毒株毒力不同等造成[18]。本研究中攻毒剂量为2 000 LD50(6×107.02 copies·只-1),攻毒1 h血液中病毒含量即达到105.04 copies·mL-1。林少莉等攻毒剂量仅为20 ELD50,Q. X. Huang等攻毒剂量为4.73×104 copies·只-1。本研究中DHAV-3在各组织中病毒含量随感染时间逐渐升高,多数于24~48 h达峰值。这与DHAV的人工感染潜伏期一般为24 h结果一致。S. L. Lin等[14]检测的200份临床死亡雏鸭样本中DHAV含量,结果表明DHAV-3感染死亡雏鸭的组织中,肝病毒含量最高(1010.42±0.71 copies·g-1),其次为脾和肾。这与本研究中结果相符,肝中病毒含量大于脾和肾,含量均在1010 copies·g-1以上。林少莉等[18]和Q. X. Huang等[15]检测结果表明DHAV-3感染的雏鸭肝中峰值时含量低于108 copies·g-1,本研究中DHAV-3感染死亡的雏鸭肝中,病毒含量高于未死亡雏鸭峰值含量,平均含量为1011.47±0.37 copies·g-1,更接近S. L. Lin等[14]检测结果。研究表明初始感染剂量越高组织中病毒含量越高。推测临床上雏鸭感染DHAV-3的剂量与本研究中攻毒剂量相近。张焕荣等[16]检测DHAV-3感染雏鸭的组织病理学和病毒分布,发现肾也是病毒侵染的重要器官之一。笔者试验中,肾剖检病变(未发表资料)和病毒含量都证明这一结论。但朱方伟等[19]建立免疫组化的方法检测DHAV-3在雏鸭组织内分布时,未在肾、心和脑中检测到,这可能与检测方法的敏感性有关。
3.3 DHAV-3致肝损伤与细胞因子变化的关联肝损伤是病毒与宿主之间相互作用的结果。先天免疫是机体第一道防线,病毒入侵后,首先被固有免疫细胞(巨噬细胞、树突状细胞等)上的模式识别受体(PRRs)识别,进而诱导产生干扰素及白介素等细胞因子。干扰素能通过激活信号通路途径诱导一系列干扰素刺激基因(ISGs)的转录,在靶细胞内建立抗病毒反应体系或者通过激活效应细胞和促进获得性免疫来调节免疫系统[32-33]。C.P. Song等[24]研究表明DHAV-1强毒和弱毒在雏鸭体内可引发不同的先天免疫反应,强弱毒均能诱导IFN表达量上调,强毒诱导IFN-γ更明显,弱毒诱导IFN-α/β更强。本研究DHAV-3能够诱导肝中Ⅰ型和Ⅱ型干扰素转录量上调,感染12 h后随病毒含量逐渐升高,48 h达到峰值,其后病毒含量下降而诱导减弱。这与C. Q. Gu等[23]研究结果不同,DHAV-1强毒在感染12 h后,干扰素α表达量受到明显抑制,仅在24 h出现短暂上调。这可能与雏鸭日龄或固有免疫水平有关。C.P. Song等[20]研究表明3周龄雏鸭拥有更强的先天免疫水平,细胞因子表达水平也较1日龄雏鸭更高。X. M. Ou等[33]研究结果也表明DHAV-1强弱毒在成年鸭上均能够引起干扰素表达量明显上调(1 000倍以上)。促炎因子可由活化的巨噬细胞产生,刺激T细胞和B细胞增殖、触发急性期反应和活化血管内皮细胞等,促进炎症反应,进而清除病毒和修复受损的组织[34]。本研究中IL-2/6在感染12 h明显上调,并与肝病变严重程度呈正比。在感染24 h达到峰值,IL-2上调1 448倍,IL-6上调132倍,此时肝组织多呈坏死性变化,细胞核固缩消失,细胞质碎裂呈碎片状或消失形成空泡,周围伴有大量易嗜性粒细胞浸润。本研究中IL-1β峰值时间要在感染后12 h,早于其他细胞因子,这提示IL-1β可能在炎症反应早期发挥重要作用。
4 结论以DHAV-3强毒感染7日龄雏鸭,发现DHAV-3在雏鸭体内具有广泛的组织嗜性,肝、脾、肾是病毒攻击的主要靶器官,肝中细胞因子可能在抑制病毒增殖和修复组织损伤过程中发挥重要作用。
| [1] | KING A M Q, ADAMS M J, CARSTENS E B, et al. Virus taxonomy:ninth report of the international committee on taxonomy of viruses[M]. Amsterdam: Elsevier, 2012: 351-373. |
| [2] | TSENG C H, TSAI H J. Molecular characterization of a new serotype of duck hepatitis virus[J]. Virus Res, 2007, 126(1-2): 19–31. DOI: 10.1016/j.virusres.2007.01.012 |
| [3] |
苏敬良, 黄瑜, 贺荣莲, 等. 新型鸭肝炎病毒的分离及初步鉴定[J]. 中国兽医科技, 2002, 32(1): 15–16.
SU J L, HUANG Y, HE R L, et al. Isolation and preliminary identification of new duck hepatitis virus[J]. Chinese Journal of Veterinary Science and Technology, 2002, 32(1): 15–16. (in Chinese) |
| [4] | WEN X J, CHENG A C, WANG M S, et al. Detection, differentiation, and VP1 sequencing of duck hepatitis A virus type 1 and type 3 by a 1-step duplex reverse-transcription PCR assay[J]. Poult Sci, 2014, 93(9): 2184–2192. DOI: 10.3382/ps.2014-04024 |
| [5] | HU Q, ZHU D K, MA G P, et al. A one-step duplex rRT-PCR assay for the simultaneous detection of duck hepatitis A virus genotypes 1 and 3[J]. J Virol Methods, 2016, 236: 207–214. DOI: 10.1016/j.jviromet.2016.07.011 |
| [6] | KIM M C, KWON Y K, JOH S J, et al. Recent Korean isolates of duck hepatitis virus reveal the presence of a new geno-and serotype when compared to duck hepatitis virus type 1 type strains[J]. Arch Virol, 2007, 152(11): 2059–2072. DOI: 10.1007/s00705-007-1023-0 |
| [7] | CHA S Y, ROH J H, KANG M, et al. Isolation and characterization of a low pathogenic duck hepatitis A virus 3 from South Korea[J]. Vet Microbiol, 2013, 162(1): 254–258. DOI: 10.1016/j.vetmic.2012.11.023 |
| [8] | DOAN H T T, LE X T K, DO R T, et al. Molecular genotyping of duck hepatitis A viruses (DHAV) in Vietnam[J]. J Infect Dev Countr, 2016, 10(9): 988–995. DOI: 10.3855/jidc.7239 |
| [9] |
黄秋雪, 汤承, 聂培婷, 等. 鸭甲肝病毒基因A型和C型双重RT-PCR检测方法的建立[J]. 中国预防兽医学报, 2012, 34(2): 120–123.
HUANG Q X, TANG C, NIE P T, et al. Establishment of a duplex RT-PCR assay for detection of duck hepatitis A virus genotype A and genotype C[J]. Chinese Journal of Preventive Veterinary Medicine, 2012, 34(2): 120–123. (in Chinese) |
| [10] | FU Y, PAN M, WANG X Y, et al. Molecular detection and typing of duck hepatitis A virus directly from clinical specimens[J]. Vet Microbiol, 2008, 131(3-4): 247–257. DOI: 10.1016/j.vetmic.2008.03.011 |
| [11] | XU Q, ZHANG R H, CHEN L L, et al. Complete genome sequence of a duck hepatitis A virus type 3 identified in eastern China[J]. J Virol, 2012, 86(24): 13848. DOI: 10.1128/JVI.02651-12 |
| [12] | CHEN L L, XU Q, ZHANG R H, et al. Improved duplex RT-PCR assay for differential diagnosis of mixed infection of duck hepatitis A virus type 1 and type 3 in ducklings[J]. J Virol Methods, 2013, 192(1-2): 12–17. DOI: 10.1016/j.jviromet.2013.04.012 |
| [13] | SOLIMAN M, ALFAJARO M M, LEE M H, et al. The prevalence of duck hepatitis A virus types 1 and 3 on Korean duck farms[J]. Arch Virol, 2015, 160(2): 493–498. DOI: 10.1007/s00705-014-2264-3 |
| [14] | LIN S L, CONG R C, ZHANG R H, et al. Circulation and in vivo distribution of duck hepatitis A virus types 1 and 3 in infected ducklings[J]. Arch Virol, 2016, 161(2): 405–416. DOI: 10.1007/s00705-015-2648-z |
| [15] | HUANG Q X, YUE H, ZHANG B, et al. Development of a real-time quantitative PCR for detecting duck hepatitis A virus genotype C[J]. J Clin Microbiol, 2012, 50(10): 3318–3323. DOI: 10.1128/JCM.01080-12 |
| [16] |
张焕容, 皮晋魁, 黄志宏. 基因C型鸭甲肝病毒实验感染雏鸭的组织病理学及病毒分布[J]. 畜牧兽医学报, 2013, 44(4): 622–628.
ZHANG H R, PI J K, HUANG Z H. Pathology and virus distribution of duck hepatitis A virus genotype C in experimentally infected ducklings[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(4): 622–628. (in Chinese) |
| [17] |
柯晨, 程安春, 汪铭书, 等. 检测鸭甲型肝炎病毒3型的免疫组织化学方法的建立和应用[J]. 中国兽医科学, 2015, 45(10): 1013–1020.
KE C, CHENG A C, WANG M S, et al. Establishment and application of immunohistochemistry for detection of duck hepatitis A virus type 3[J]. Chinese Veterinary Science, 2015, 45(10): 1013–1020. (in Chinese) |
| [18] |
林少莉, 杨蕾, 张瑞华, 等. 鸭甲肝病毒3型荧光定量PCR检测方法的建立及其在雏鸭体内分布规律[J]. 中国兽医学报, 2016, 36(5): 723–727, 733.
LIN S L, YANG L, ZHANG R H, et al. Development of a real-time quantitative PCR for detection and investigation of in vivo dynamic distribution of duck hepatitis virus type 3[J]. Chinese Journal of Veterinary Science, 2016, 36(5): 723–727, 733. (in Chinese) |
| [19] |
朱方伟, 谷长勤, 程国富, 等. 新型鸭肝炎病毒感染雏鸭组织内病毒抗原的分布[J]. 华中农业大学学报, 2007, 26(4): 511–513.
ZHU F W, GU C Q, CHENG G F, et al. Dynamic distribution of new type duck hepatitis virus in experimental infectious duckling[J]. Journal of Huazhong Agricultural University, 2007, 26(4): 511–513. (in Chinese) |
| [20] | SONG C P, YU S Q, DUAN Y B, et al. Effect of age on the pathogenesis of DHV-1 in Pekin ducks and on the innate immune responses of ducks to infection[J]. Arch Virol, 2014, 159(5): 905–914. DOI: 10.1007/s00705-013-1900-7 |
| [21] |
柯晨. 检测3型鸭甲肝病毒免疫组化方法建立和应用[D]. 成都: 四川农业大学, 2015.
KE C. Establishment and application of immunohistochemistry to detect duck hepatitis A virus type 3[D]. Chengdu:Sichuan Agricultural University, 2015. (in Chinese) |
| [22] | WANG X Y, ZHANG J J, MENG R Z, et al. Host differences affecting resistance and susceptibility of the second generation of a pekin duck flock to duck hepatitis A virus genotype 3[J]. Front Microbiol, 2017, 8: 1128. DOI: 10.3389/fmicb.2017.01128 |
| [23] | GU C Q, XIE C Q, HU X Y, et al. Cytokine gene expression in the livers of ducklings infected with duck hepatitis virus-1 JX strain[J]. Poult Sci, 2012, 91(3): 583–591. DOI: 10.3382/ps.2011-01743 |
| [24] | SONG C P, LIAO Y, GAO W, et al. Virulent and attenuated strains of duck hepatitis A virus elicit discordant innate immune responses in vivo[J]. J Gen Virol, 2014, 95(12): 2716–2726. |
| [25] | MAO S, OU X M, ZHU D K, et al. Development and evaluation of indirect ELISAs for the detection of IgG, IgM and IgA1 against duck hepatitis A virus 1[J]. J Virol Methods, 2016, 237: 79–85. DOI: 10.1016/j.jviromet.2016.08.019 |
| [26] | OU X M, MAO S, JIANG Y F, et al. Viral-host interaction in kidney reveals strategies to escape host immunity and persistently shed virus to the urine[J]. Oncotarget, 2017, 8(5): 7336–7349. |
| [27] | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the method[J]. Methods, 2001, 25(4): 402–408. DOI: 10.1006/meth.2001.1262 |
| [28] |
杨发龙, 张焕容, 程方明, 等. 基于基因C型鸭甲肝病毒VP1重组蛋白的鸭甲肝病毒抗体ELISA检测方法的建立[J]. 畜牧兽医学报, 2014, 45(7): 1148–1153.
YANG F L, ZHANG H R, CHENG F M, et al. Development of an indirect ELISA for detecting antibodies against Genotype A and C duck hepatitis A virus based on recombinant VP1 protein[J]. Acta Veterinaria et Zootechnica Sinica, 2014, 45(7): 1148–1153. (in Chinese) |
| [29] |
张玉瑶, 马秀丽, 黄兵, 等. 鸭甲肝病毒1型和3型间接ELISA方法的建立与应用[J]. 微生物学报, 2015, 55(4): 501–509.
ZHANG Y Y, MA X L, HUANG B, et al. Indirect ELISA for simultaneous detection of antibodies against duck hepatitis A type 1 and 3 viruses[J]. Acta Microbiologica Sinica, 2015, 55(4): 501–509. (in Chinese) |
| [30] | SUN X Y, DIAO Y X, WANG J, et al. Tembusu virus infection in Cherry Valley ducks:the effect of age at infection[J]. Vet Microbiol, 2014, 168(1): 16–24. DOI: 10.1016/j.vetmic.2013.10.003 |
| [31] | LI N, LV C W, YUE R C, et al. Effect of age on the pathogenesis of duck tembusu virus in Cherry Valley ducks[J]. Front Microbiol, 2015, 6: 581. |
| [32] | RANDALL R E, GOODBOURN S. Interferons and viruses:an interplay between induction, signalling, antiviral responses and virus countermeasures[J]. J Gen Virol, 2008, 89(Pt 1): 1–47. |
| [33] | OU X M, MAO S, CAO J Y, et al. Comparative analysis of virus-host interactions caused by a virulent and an attenuated duck hepatitis A virus genotype 1[J]. PLoS One, 2017, 12(6): e0178993. DOI: 10.1371/journal.pone.0178993 |
| [34] | GIANSANTI F, GIARDI M F, BOTTI D. Avian cytokines-an overview[J]. Curr Pharm Des, 2006, 12(24): 3083–3099. DOI: 10.2174/138161206777947542 |



