2. 河北省畜牧兽医研究所, 保定 071000
2. Institute of Animal Science and Veterinary Medicine of Hebei, Baoding 071000, China
肌肉生长抑制素(Myostatin, MSTN),又称之为GDF-8,属于转化生长因子-β(Transforming growth factor-β, TGF-β)超家族成员,是能够在骨骼肌中特异表达的一类糖蛋白,负向调控肌肉的生长和发育[1]。生肌过程是一个复杂的过程,在早期胚胎中来自于生肌节区的中胚层细胞转移至胚性结缔组织附近,生成骨骼肌成肌细胞。成肌细胞增殖后,彼此融合分化形成多核的肌纤维[2]。
生肌调节因子(MRFs)家族,包括生肌决定因子(Myogenic factor 5, Myf5)、成肌分化因子(Myogenic differentiation antigen, MyoD)、肌细胞生成素(Myogenin, MyoG)和Myf6即肌调节因子4(Muscle regulatory factor 4, MRF4)等4个转录因子,参与骨骼肌生成过程中分子调控机制,启动和维持骨骼肌细胞分化发育和生长[3]。研究发现,Myf5和MyoD可以使成肌细胞定型[4],而MyoG和Myf6在成肌细胞的融合和分化中起作用[5]。细胞周期抑制因子(p21)与细胞增殖密切相关。羟酰基辅酶A脱氢酶1(Hydroxyacyl CoA dehydratase 1, HACD1)是细胞膜组成和流动的调节因子,在肌肉发育和再生中促进成肌细胞融合[6]。
以往的报道大多集中研究干扰MSTN后相关基因的表达[7-11],本试验通过腺病毒介导shRNA干扰绵羊成肌细胞MSTN,研究MSTN基因对绵羊成肌细胞增殖和分化的影响,探讨其作用机制,确定绵羊MSTN基因与p21基因在增殖中的表达调控关系,及其与生肌调节因子家族MRFs和HACD1基因在分化过程中的表达调控关系,为深入了解MSTN基因对绵羊肌肉生长发育的作用及其调控机制奠定理论基础。
1 材料与方法 1.1 材料小尾寒羊成肌细胞由本实验室分离纯化[12],shRNA重组腺病毒阴性对照载体NC和干扰载体Sh3+4按文献[13]所示方法构建,由本实验室保存(经qRT-PCR检测干扰效率达到72%,经Western blot检测干扰效率达到54%),胎牛血清(FBS)、DMEM/F12培养基、胰蛋白酶、DPBS缓冲液、青链霉素混合液(100×)购自BI公司,反转录试剂盒(PrimeScript® RT)购自宝生物工程(大连)有限公司,Bestar SybrGreen qPCR Mastermix购自德国DBI公司。
1.2 成肌细胞的培养及转染成肌细胞为本实验室冻存的第2代细胞,解冻后用培养液(90%DMEM/F12+10%FBS)培养。转染前24 h,将细胞接种到培养板中,使转染时细胞密度达到80%左右。阴性对照载体NC、干扰载体Sh3+4腺病毒用培养液稀释100倍转染细胞,6孔培养板中每孔加入1 mL病毒稀释液。感染5 h后进行换液,24 h后在倒置荧光显微镜下观察细胞生长状况及绿色荧光蛋白表达情况。
1.3 成肌细胞的增殖 1.3.1 CCK-8检测成肌细胞增殖成肌细胞感染腺病毒2 d后,利用0.25%胰蛋白酶消化后制成细胞悬液,96孔板每孔加入2 000个细胞,重复3孔,每天在同一时间点取出培养板进行检测,每孔加入10 μL CCK-8溶液,在细胞培养箱内继续孵育2.5 h,在酶标仪震荡后以450 nm波长测定吸光度,连续测定7 d,绘制细胞生长曲线。
1.3.2 流式细胞仪(PI染色法)检测细胞周期成肌细胞感染腺病毒2 d后,用不含EDTA的胰酶消化细胞制成单细胞悬液,1 000 r·min-1离心5 min,去上清液。用预冷的PBS洗细胞2次,加入预冷的70%乙醇,于4℃固定过夜。离心弃上清,用PBS洗1次,加入500 μL的PI/RNase溶液重悬,避光室温孵育15 min。每组3个重复。FACS Calibur流式细胞仪采用ModFit LT软件进行细胞DNA含量分析。
1.3.3 qRT-PCR检测p21基因的表达成肌细胞感染病毒48 h后,荧光倒置显微镜下观察绿色荧光蛋白表达情况,收集细胞,用Trizol法提取各组细胞总RNA,电泳检测质量,Nanodrop 2000超微量分光光度计定量后,按照TaKaRa(PrimeScriptTM RT Reagent Kit)逆转录试剂盒操作程序进行反转录合成cDNA第一条链,再以cDNA为模板,采用SYBR GreenI法进行qRT-PCR测定,每组3个重复。PCR反应体系:SybrGreen qPCR Master Mix 10 μL,正、反向引物各0.5 μL,ROX Reference Dye 0.4 μL,cDNA模板1.0 μL,ddH2O加至20 μL。反应程序:95 ℃预变性1 min;95 ℃变性15 s,60 ℃退火延伸60 s并收集荧光信号,共40个循环;熔解曲线分析:95 ℃15 s,60~95 ℃,0.3 ℃·s-1生成熔解曲线。以GAPDH为内参基因,2-ΔΔCt法进行相对定量计算,每个样品重复3次,取平均值。
1.4 成肌细胞的分化 1.4.1 成肌细胞融合率测定将感染腺病毒2 d的细胞接种到细胞爬片,用2%马血清诱导分化到第3天,进行细胞免疫组化染色。PBS清洗标本3次,每次2 min;用4%多聚甲醛固定细胞15 min,0.3% TritonX-100穿孔20 min,3%双氧水孵育5 min,10%驴血清37 ℃封闭20 min,滴加一抗MYH1(1:200稀释度)4 ℃过夜。PBS缓冲液洗3次。滴加PV-6000,37 ℃孵育30 min,PBS漂洗3次,每次5 min;DAB显色,DAPI染色3 min。荧光显微镜下观察染色后的肌管形态,随机选取3个视野,使用NIS-Elements(Nikon)软件计量视野内融合到多核细胞内的细胞核的数目,除以整个视野中细胞核数目,计算其百分数即为细胞融合率。
1.4.2 qRT-PCR检测成肌细胞分化后相关基因的表达成肌细胞感染腺病毒2 d后,换含2%马血清的培养基对成肌细胞进行诱导分化,分别于诱导分化后的48、72、96 h收集细胞,提取RNA。测定MSTN、MyoD、MyoG、Myf5、Myf6和HACD1基因的表达情况。引物序列见表 1。
|
|
表 1 基因引物参数 Table 1 Parameters of primer pairs for the genes |
采用SPASS19.0统计软件进行分析,组间比较利用单因素方差独立样本t检验进行方差分析和显著性检验。试验数据以“平均值±标准差”表示,P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果 2.1 干扰MSTN后对绵羊成肌细胞增殖的影响CCK-8检测结果显示(图 1),培养1~3 d两组OD值几乎相同,细胞活性无显著性差异(P > 0.05),但在第3天后干扰组细胞生长明显减缓,第4天时OD值比阴性对照组低(19.3±0.04)%,第5天时低(38.2±0.03)%,第6天时低(44.3±0.03)%,第7天时低(21.9±0.12)%,差异均达到显著水平(P < 0.05),第6天为极显著(P < 0.01),说明干扰MSTN后成肌细胞增殖能力降低。
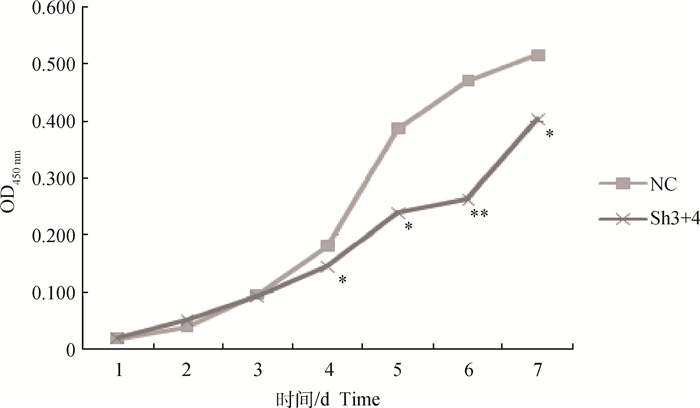
|
与对照组相比,**表示差异极显著(P < 0.01),*表示差异显著(P < 0.05)。下同 Compared with the negative control group, ** indicate extremely significant difference (P < 0.01), * indicate significant difference (P < 0.05). The same as below 图 1 成肌细胞生长曲线 Figure 1 The growth curve of myoblast cells |
成肌细胞感染腺病毒48 h后进行细胞周期检测(图 2,表 2),相对于阴性对照组,干扰组处于G0/G1期细胞的比例显著增加(P < 0.05),而S期细胞所占的比例显著下降(P < 0.05)。表明干扰内源性MSTN后抑制绵羊成肌细胞增殖。
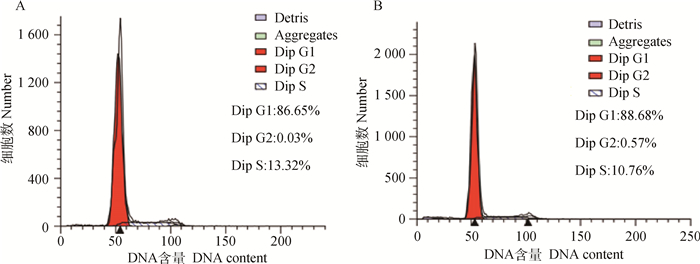
|
A. NC; B. Sh3+4 图 2 流式细胞图 Figure 2 Flow cytometry |
|
|
表 2 干扰MSTN后细胞周期变化 Table 2 The cell cycle alteration after MSTN interfered |
干扰MSTN后p21基因的表达见图 3,结果表明,Sh3+4干扰组较阴性对照组p21的mRNA表达量升高了1.42倍,达到极显著水平(P < 0.01)。
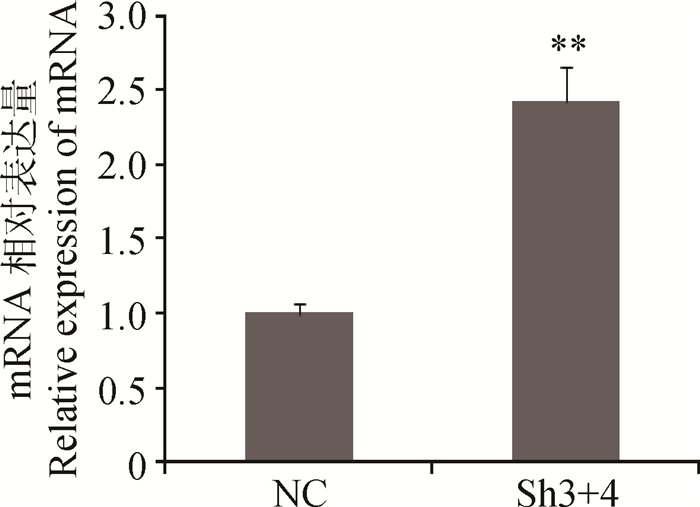
|
图 3 干扰MSTN后p21基因的表达 Figure 3 The expression of p21 after of MSTN interfered |
成肌细胞经重组腺病毒感染后进行诱导分化,在诱导48~96 h时,相较于阴性对照,干扰组Sh3+4细胞的MSTN基因表达量均极显著减少(P < 0.01,图 4),96 h时达到最低值,仅为对照组细胞的10%,达到极显著水平(P < 0.01),表明Sh3+4腺病毒对MSTN表达进行了持续有效的干扰。
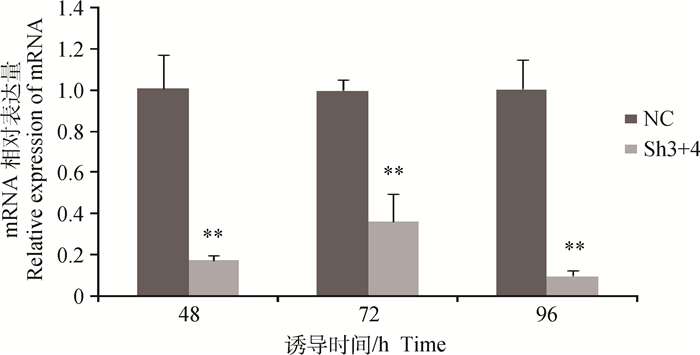
|
图 4 成肌细胞分化后MSTN基因的表达 Figure 4 The expression of MSTN gene after myoblast differentiating |
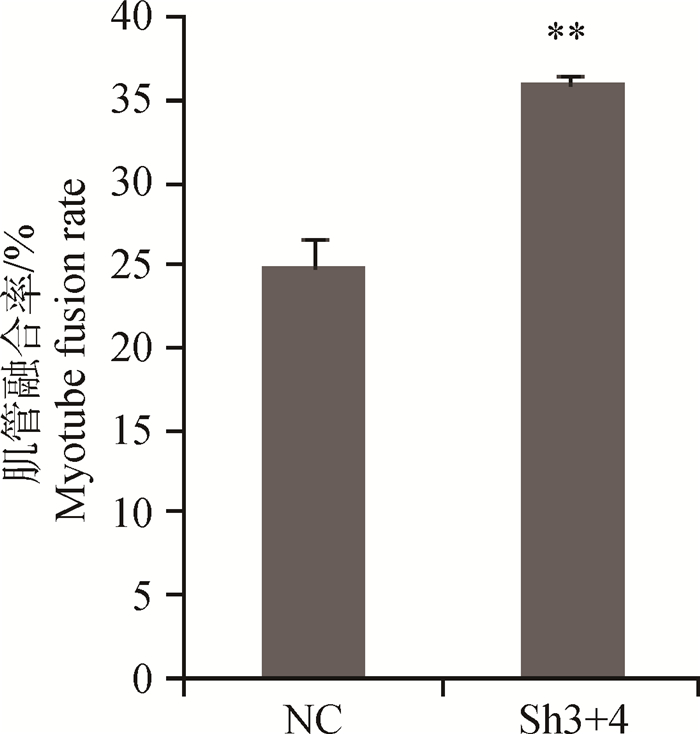
腺病毒干扰载体感染的成肌细胞在含2%马血清的培养基中培养,第3天后显微镜下观察,当细胞汇合度达到80%时,相邻成肌细胞靠拢,由长梭形变得细长,呈平行排列走向,细胞膜模糊,含多个细胞核,呈现出长条管状结构,即肌小管。进行免疫细胞化学染色(图 5),测定分化指标肌管融合率,相对于阴性对照组,Sh3+4干扰组肌管融合率增加了43.6%, 达到极显著水平(P < 0.01,图 6)。
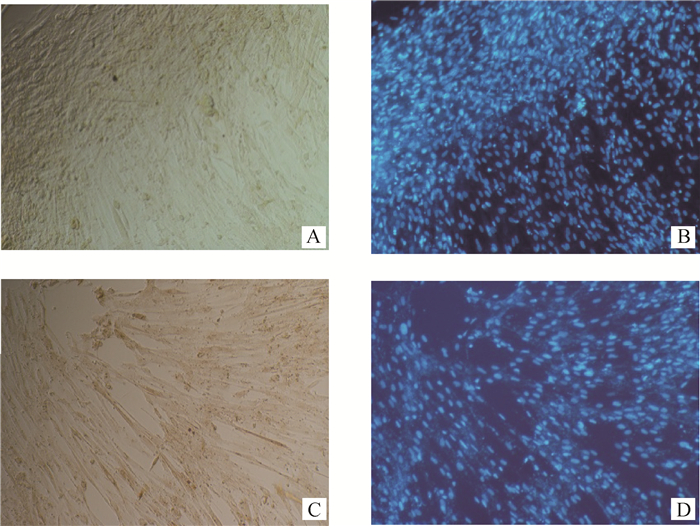
|
A.阴性对照(NC)组免疫细胞化学染色;B.阴性对照(NC)组DAPI核染;C. Sh3+4干扰组免疫细胞化学染色;D. Sh3+4干扰组DAPI核染 A.Negative control group (NC) stained by immunocytochemistry; B. Negative control group (NC) stained by DAPI; C. Interference group Sh3+4 stained by immunocytochemistry; D. Interference group Sh3+4 stained by DAPI 图 5 成肌细胞分化72 h后MYH1免疫细胞化学结果(100×) Figure 5 Myoblasts stained with anti-MYH1 antibody at 72 h in differentiation medium (100×) |
|
图 6 成肌细胞肌管融合率 Figure 6 Myotube fusion rate of myoblast |
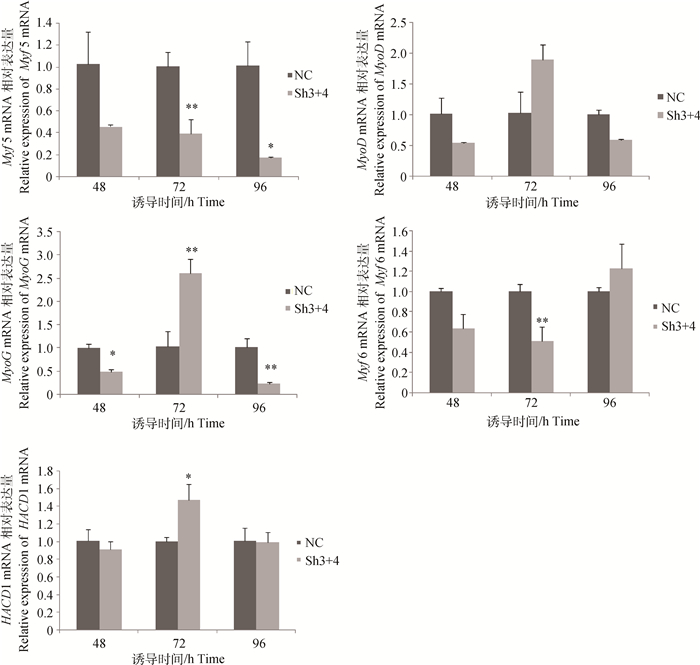
qRT-PCR检测生肌调节因子及细胞融合相关基因HACD1的mRNA表达水平见图 7。Sh3+4干扰组与NC阴性对照组相比,诱导48 h后4个生肌调节因子表达量均呈下降趋势,MyoG基因达到显著水平(P < 0.05);诱导72 h后 Myf5和Myf6基因表达量极显著降低(P < 0.01),MyoD基因表达量升高不显著(P > 0.05),MyoG基因极显著增加(P < 0.01),说明此时与阴性对照组相比在Sh3+4干扰组有更多的成肌细胞融合为肌管;诱导96 h,Myf5基因表达量显著降低(P < 0.05),MyoD基因表达量降低不显著(P > 0.05),MyoG基因表达量极显著降低(P < 0.01),Myf6基因表达量升高不显著(P > 0.05)。另外诱导72 h,HACD1基因的表达量在干扰组中显著高于阴性对照组(P < 0.05),诱导48和96 h无显著变化,证实了该基因与成肌细胞融合相关。
|
图 7 成肌细胞诱导分化后调控基因的表达 Figure 7 Regulatory genes expression after myoblasts differentiating |
肌肉质量的增加包括肌纤维增生和肥大,该过程包括成肌细胞的增殖和分化。早期肌肉祖细胞被特化,退出细胞周期,最终分化成肌纤维[14]。深入了解分化过程中的关键环节,进一步探索肌肉的生长发育机制对提升家畜动物的产肉性能具有十分重要的意义。大多数报道认为,MSTN负调控肌源性细胞的增殖[15-19],但D.Joulia等[20]的研究发现,抑制C2C12成肌细胞内源性MSTN后增强了细胞周期的退出从而刺激细胞分化。B.D.Rodgers等[21]对C2C12的研究发现,MSTN对细胞的生长有激活作用。MSTN在肌源性细胞分化中的作用也存在争议,一些研究证明,在不同的物种中其对成肌细胞的分化有抑制作用[16, 22-24],而其他研究发现MSTN对肌源性细胞的分化有刺激促进作用[25-28]。本试验通过腺病毒介导shRNA抑制内源性MSTN后,研究成肌细胞的增殖和分化,结果并未观察到干扰MSTN基因促进成肌细胞的增殖,生长曲线第3天后反而显示增殖能力降低。此结果与p21基因表达升高,细胞生长周期停滞在G0/G1的结果吻合。p21是细胞周期抑制因子基因,该基因的上调可抑制cyclin-Cdk复活物活力,使Rb蛋白不能磷酸化而呈现低磷酸化状态。S期基因表达所必需的转录因子(E2F-DP1)不能释放,细胞停滞在G1期,从而导致成肌细胞增殖抑制,数量减少。干扰MSTN后抑制成肌细胞生长的结果与大多数报道不一致的原因:一可能是本研究采用的是绵羊成肌细胞,以往报道多集中在小鼠、山羊等物种;二可能是本研究采用的RNA干扰介导载体为腺病毒,以往大多采用siRNA、质粒或是慢病毒载体;三可能是在生长后期干扰组成肌细胞被触发分化而抑制增殖。本研究发现,干扰MSTN基因后成肌细胞的融合率显著增加,与大多数报道一致。因此MSTN对成肌细胞增殖及分化的确切作用有待进一步验证。
本试验检测了成肌细胞诱导分化后参与肌肉分化的生肌调节因子Myf5、MyoD、MyoG和Myf6基因表达的变化,这些因子的时序表达对于确定肌源性是必需的,Myf5在静止和激活的卫星细胞中表达,MyoD和MyoG是下游分化因子,Myf6在肌管成熟过程中起作用[29]。本研究发现,诱导72 h时MyoD和MyoG基因的表达量在干扰组比阴性对照组高,同时还发现在诱导72 h时,肌管有大量的融合,在干扰组中有更多的肌细胞生成肌管,表明抑制MSTN基因后对细胞的分化起到了促进作用。此前W.Luo等[30]的研究证实MyoD和MyoG通过结合到Myomaker启动子上促进成肌细胞分化。本研究发现,诱导72 h时,Myf5基因的表达量在干扰组比对照组低,进一步证实了Myf5是成肌细胞早期表达的基因,可能不参与肌管形成的末期。诱导96 h时,Myf6基因的表达量在干扰组比阴性对照组高,暗示在干扰组中有更多的肌纤维形成,也进一步说明了Myf6在肌管成熟后期起作用。本研究进一步证实,相关基因在分化过程中依照Myf5、MyoD、MyoG和Myf6的顺序依次表达。
本研究检测了与细胞融合相关的HACD1基因,HACD1基因是膜组成和流动性的调节因子,在72 h时干扰组肌管融合率极显著高于阴性对照组,并且HACD1基因表达量显著高于对照组,证实了该基因与成肌细胞融合密切相关,此结果与J.Blondelle等[6]的研究结果一致。
4 结论本研究证实MSTN对成肌细胞增殖有促进作用,对成肌细胞的分化融合有抑制作用。确定了在成肌细胞分化过程中MSTN基因与Myf5、MyoD、MyoG和Myf6的表达调控关系。进一步证实HACD1基因与成肌细胞融合相关。本研究为深入揭示MSTN基因对绵羊肌肉生长发育的作用及其调控机制奠定了理论基础。
| [1] | MCPHERRON A C, LAWLER A M, LEE S J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member[J]. Nature, 1997, 387(6628): 83–90. DOI: 10.1038/387083a0 |
| [2] | YAFFE D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells[J]. Proc Natl Acad Sci U S A, 1968, 61(2): 477–483. DOI: 10.1073/pnas.61.2.477 |
| [3] |
高丽, 郑陆. 肌肉生长抑制素GDF-8[J]. 山东体育学院学报, 2004, 20(3): 45–47.
GAO L, ZHENG L. Myostatin, a negative regulator of muscle growth[J]. Journal of Shandong Institute of Physical Education, 2004, 20(3): 45–47. (in Chinese) |
| [4] | CAMPBELL J L Jr, SMITH M A, FISHER J W, et al. Dose-response for retinoic acid-induced forelimb malformations and cleft palate:A comparison of computerized image analysis and visual inspection[J]. Birth Defects Res B Dev Reprod Toxicol, 2004, 71(4): 289–295. DOI: 10.1002/(ISSN)1542-9741 |
| [5] | YU Z L, LIN J X, XIAO Y, et al. Induction of cell-cycle arrest by all-trans retinoic acid in mouse embryonic palatal mesenchymal (MEPM) cells[J]. Toxicol Sci, 2005, 83(2): 349–354. |
| [6] | BLONDELLE J, OHNO Y, GACHE V, et al. HACD1, a regulator of membrane composition and fluidity, promotes myoblast fusion and skeletal muscle growth[J]. J Mol Cell Biol, 2015, 7(5): 429–440. DOI: 10.1093/jmcb/mjv049 |
| [7] | LU J, SUN D, XU L Y, et al. Selection of an effective small interference RNA to silence myostatin gene expression in sheep fibroblast cells[J]. Biochem Genet, 2012, 50(11-12): 838–847. DOI: 10.1007/s10528-012-9524-2 |
| [8] | LU J, WEI C H, ZHANG X N, et al. The effect of myostatin silencing by lentiviral-mediated RNA interference on goat fetal fibroblasts[J]. Mol Biol Rep, 2013, 40(6): 4101–4108. DOI: 10.1007/s11033-013-2494-6 |
| [9] | PATEL A K, TRIPATHI A K, PATEL U A, et al. Myostatin knockdown and its effect on myogenic gene expression program in stably transfected goat myoblasts[J]. In Vitro Cell Dev Biol Anim, 2014, 50(7): 587–596. DOI: 10.1007/s11626-014-9743-4 |
| [10] | KUMAR R, SINGH S P, KUMARI P, et al. Small interfering RNA (siRNA)-mediated knockdown of myostatin influences the expression of myogenic regulatory factors in caprine foetal myoblasts[J]. Appl Biochem Biotechnol, 2014, 172(3): 1714–1724. DOI: 10.1007/s12010-013-0582-7 |
| [11] | PATEL U A, PATEL A K, JOSHI C G. Stable suppression of myostatin gene expression in goat fetal fibroblast cells by lentiviral vector-mediated RNAi[J]. Biotechnol Prog, 2015, 31(2): 452–459. DOI: 10.1002/btpr.v31.2 |
| [12] |
王红娜, 张英杰, 刘月琴. 绵羊成肌细胞的纯化、培养、鉴定及其成肌诱导分化研究[J]. 河北农业大学学报, 2016, 39(1): 94–98, 102.
WHONG H N, ZHANG Y J, LIU Y Q. A study of purification, culture, identification and differentiation of sheep myoblast cells[J]. Journal of Agricultural University of Hebei, 2016, 39(1): 94–98, 102. (in Chinese) |
| [13] |
孔庆辉, 晁燕, 夏明哲, 等. 黄河裸裂尻鱼MSTN基因RNA干扰研究[J]. 中国实验动物学报, 2016, 24(4): 344–350.
KONG Q H, CHAO Y, XIA M Z, et al. Inhibitory effect of RNA interference of MSTN gene expression on the downstream genes in Schizopygopsis pylzovi[J]. Acta Laboratorium Animalis Scientia Sinica, 2016, 24(4): 344–350. (in Chinese) |
| [14] | KITZMANN M, FERNANDEZ A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts[J]. Cell Mol Life Sci, 2001, 58(4): 571–579. DOI: 10.1007/PL00000882 |
| [15] | THOMAS M, LANGLEY B, BERRY C, et al. Myostatin, a negative regulator of muscle growth, functions by Inhibiting myoblast proliferation[J]. J Biol Chem, 2000, 275(51): 40235–40243. DOI: 10.1074/jbc.M004356200 |
| [16] | RÍOS R, CARNEIRO I, ARCE V M, et al. Myostatin is an inhibitor of myogenic differentiation[J]. Am J Physiol Cell Physiol, 2002, 282(5): C993–C999. DOI: 10.1152/ajpcell.00372.2001 |
| [17] | LANGLEY B, THOMAS M, MCFARLANE C, et al. Myostatin inhibits rhabdomyosarcoma cell proliferation through an Rb-independent pathway[J]. Oncogene, 2004, 23(2): 524–534. DOI: 10.1038/sj.onc.1207144 |
| [18] |
孙顺昌, 彭运生, 贺敬波, 等. siRNA阻断鼠成肌细胞myostatin表达对细胞增殖及分化能力的影响[J]. 基础医学与临床, 2011, 31(2): 187–191.
SUN S C, PENG Y S, HE J B, et al. Effect of myostatin silence mediated by mouse siRNA on cell proliferation and differentiation in myoblasts[J]. Basic & Clinical Medicine, 2011, 31(2): 187–191. (in Chinese) |
| [19] | KUMAR R, SINGH S P, MITRA A. Short-hairpin mediated myostatin knockdown resulted in altered expression of myogenic regulatory factors with enhanced myoblast proliferation in fetal myoblast cells of goats[J]. Anim Biotechnol, 2017, 30: 1–9. |
| [20] | JOULIA D, BERNARDI H, GARANDEL V, et al. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin[J]. Exp Cell Res, 2003, 286(2): 263–275. DOI: 10.1016/S0014-4827(03)00074-0 |
| [21] | RODGERS B D, WIEDEBACK B D, HOVERSTEN K E, et al. Myostatin stimulates, not inihibits, C2C12 myoblast proliferation[J]. Endocrinology, 2014, 155(3): 670–675. DOI: 10.1210/en.2013-2107 |
| [22] | LANGLEY B, THOMAS M, BISHOP A, et al. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression[J]. J Biol Chem, 2002, 277(51): 49831–49840. DOI: 10.1074/jbc.M204291200 |
| [23] | MCFARLANE C, HUI G Z, AMANDA W Z, et al. Human myostatin negatively regulates human myoblast growth and differentiation[J]. Am J Physiol Cell Physiol, 2011, 301(1): C195–C203. DOI: 10.1152/ajpcell.00012.2011 |
| [24] | LIU C X, LI W R, ZHANG X M, et al. The critical role of myostatin in differentiation of sheep myoblasts[J]. Biochem Biophys Res Commun, 2012, 422(3): 381–386. DOI: 10.1016/j.bbrc.2012.04.151 |
| [25] | SATO F, KUROKAWA M, YAMAUCHI N, et al. Gene silencing of myostatin in differentiation of chicken embryonic myoblasts by small interfering RNA[J]. Am J Physiol Cell Physiol, 2006, 291(3): C538–C545. DOI: 10.1152/ajpcell.00543.2005 |
| [26] | MANCEAU M, GROS J, SAVAGE K, et al. Myostatin promotes the terminal differentiation of embryonic muscle progenitors[J]. Genes Dev, 2008, 22(5): 668–681. DOI: 10.1101/gad.454408 |
| [27] | GARIKIPATI D K, RODGERS B D. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation:evidence for endocrine-regulated transcript processing[J]. J Endocrinol, 2012, 215(1): 177–187. DOI: 10.1530/JOE-12-0260 |
| [28] | WEI C, REN H, XU L, et al. Signals of Ezh2, Src, and Akt Involve in myostatin-Pax7 pathways regulating the myogenic fate determination during the sheep myoblast proliferation and differentiation[J]. PLoS ONE, 2015, 10(3): e0120956. DOI: 10.1371/journal.pone.0120956 |
| [29] | SABOURIN L A, RUDNICKI M A. The molecular regulation of myogenesis[J]. Clin Genet, 2000, 57(1): 16–25. |
| [30] | LUO W, LI E X, NIE Q H, et al. Myomaker, regulated by MYOD, MYOG and miR-140-3p, promotes chicken myoblast fusion[J]. Int J Mol Sci, 2015, 16(11): 26186–26201. DOI: 10.3390/ijms161125946 |



