伏马毒素(fumonisin)主要由串珠镰刀菌和再育镰刀菌等真菌产生,是一类霉菌毒素的统称,包含多种毒素及其衍生物,以B族伏马菌素最为常见[1]。伏马毒素是一种单端孢酶烯族化合物,广泛分布于世界各地的各种粮食作物中[2],是目前谷物污染的主要霉菌毒素之一,其性质非常稳定,在畜禽产出的肉、蛋、奶等产品中均能检测到毒素存在[3]。
伏马毒素对人和多种动物都有毒性作用,具有广泛毒性。伏马毒素对人体的危害主要是引起食管癌和神经管型缺陷病[4-5]。南非、伊朗和中国的人群流行病学调查资料表明食管癌高发区居民粮食中FB1的污染程度高于低发区居民[6-7]。畜禽摄入伏马毒素后,会损害消化系统、免疫系统、生殖系统、神经内分泌系统等[8],诱导呕吐、腹泻、出血、出现免疫抑制[9-10],延长种畜的性成熟期[11],引起胎儿畸形[12],降低饲料转化率,降低生产性能。因此,伏马毒素的污染,对人类健康和畜禽养殖业造成严重的危害。基于细胞水平的研究表明伏马毒素对人和小鼠的多种细胞有毒性作用,但是具体的分子机制尚不清楚[13-15]。作为体内的循环系统,血管组织最广泛、最容易受到毒素的侵害,但是FB1对血管内皮细胞的毒性作用研究尚未见报道。因此,本试验以人脐静脉血管内皮细胞(human umbilical vein epithelial cells,HUVEC)作为供试细胞,研究伏马毒素对HUVEC的毒性作用,旨在进一步探讨伏马毒素的毒性作用机制、与人畜疾病的确切关系,为我国食品和饲料中伏马毒素限量标准的研制、正确指导农业生产、开展风险评估、污染预警和防控霉菌毒素中毒提供理论基础。
1 材料与方法 1.1 主要试剂DMEM-H干粉培养基、胎牛血清、青霉素/链霉素溶液(100×)和胰蛋白酶等均购自Gbico公司。细胞增殖检测试剂盒(CCK-8法)、细胞周期检测试剂盒、BCA蛋白质浓度测定试剂盒等均购自上海碧云天生物科技有限公司。TRIZOL试剂购自Invitrogen公司。反转录试剂盒和荧光定量试剂盒购自东洋纺(上海)生物科技有限公司。伏马毒素B1(FB1)购自美国Sigma公司。人脐静脉血管内皮细胞(HUVEC)购自上海博谷生物科技有限公司。其余试剂为进口或国产分析纯。
1.2 FB1对HUVEC细胞形态和活力的影响HUVEC按3.5×104·cm-2的细胞密度接种至6孔板中。当细胞生长至60%~70%融合时,弃培养基,加入含不同质量浓度FB1(FB1终质量浓度分别为2.5、5、10和20 μg·mL-1)的培养基。以不加FB1细胞作为对照组,继续培养24和48 h后,分别在倒置显微镜下观察拍照。参照细胞活力检测试剂盒(CCK-8法)说明书,检测FB1处理组和对照组的细胞活力。按以下公式计算细胞活力:细胞活力百分比=(FB1处理组OD值-空白组OD值)/(对照组OD值-空白组OD值)×100%。
1.3 FB1对HUVEC细胞周期的影响细胞接板和药物处理方法同“1.2”,继续培养24和48 h后,按照细胞周期检测试剂盒说明书步骤操作,准备好细胞样品,进行流式细胞仪检测。上述试验重复3次。
1.4 FB1对HUVEC中活性氧水平的影响HUVEC按3×104·cm-2的细胞密度接种至12孔板中。当细胞生长至80%左右融合时,弃培养基,加入含不同质量浓度FB1(FB1终质量浓度分别为5、10和20 μg·mL-1)的培养基。以不加FB1细胞为对照组,继续培养48 h后,加入含DCFH-DA荧光染料的培养液,37 ℃避光孵育40 min,洗涤3次后进行荧光倒置显微镜观察,并拍照。
1.5 FB1对HUVEC线粒体膜电位的影响细胞接板和药物处理方法同“1.2”,继续培养48 h后,加入含JC-1荧光染料的培养液,37 ℃避光孵育30 min,用PBS洗涤2次,荧光倒置显微镜观察并拍照。同时用准备好的同批次细胞制备样品进行流式细胞仪检测。
1.6 FB1对HUVEC Hoechst33342染色效果的影响细胞接板和药物处理方法同“1.2”,继续培养48 h后,用无血清培养基洗涤两次,加入Hoechst-33342染液,37 ℃避光孵育15 min,洗涤3次后进行荧光倒置显微镜观察,并拍照。
1.7 引物的设计与合成根据GenBank上已登录的人Caspase-3、Caspase-9、Bax和Bcl-2基因的全基因序列,利用Oligo 7.0软件设计4对特异性引物。引物由英潍捷基贸易有限公司合成。Caspase-3基因上、下游引物的序列分别为5′-ATGCTGAAACAGTATGCCGACAA-3′, 5′-GCGTCAAAGGAAAAGGA CTCAAAT-3′,扩增片段为98 bp;Caspase-9基因上、下游引物的序列分别为5′-AGCCAACCCTAGAAAACCTTACC-3′,5′-TCACCAAATCCTCCAGAACCAAT-3′,扩增片段为115 bp;Bax基因上、下游引物的序列分别为5′-CCCCGAGAGGTCTTTTTCCGAG-3′,5′-AGGGCCTTGAGCA CCAGTTTG-3′,扩增片段为113 bp;Bcl-2基因上、下游引物的序列分别为5′-GTGCCTGCTTTTAGGAGACCGA-3′,5′-GAGACCACACTGCCCTGTTGATC-3′,扩增片段为128 bp。
1.8 FB1处理对HUVEC凋亡相关基因转录的影响细胞接板和药物处理方法同“1.2”,继续培养48 h后,采用TRIZOL法提取总RNA,参照反转录试剂盒说明书反转录出cDNA,实时荧光定量PCR检测细胞凋亡相关基因的转录情况。
1.9 统计分析采用SPSS17.0统计软件对所有试验数据进行统计分析,采用单因素组间比较,配对学生t检验,各指标均以“ x±s”表示,以P<0.05表示差异显著,P<0.01表示差异极显著。
2 结果 2.1 FB1对HUVEC形态和活力的影响FB1对HUVEC形态的影响结果见图 1。由图 1可见,与对照组相比,处理组细胞形态欠规则、呈圆形或卵圆形、折光性差、细胞质内黑色颗粒物质增多、贴壁细胞减少,悬浮的死细胞增多。
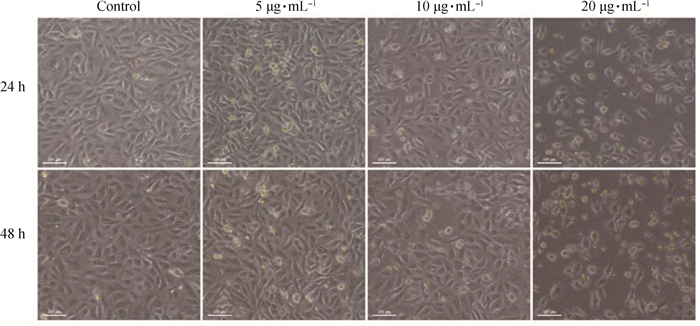
|
图 1 不同浓度FB1作用对HUVEC细胞生长的观察(标尺=100 μm) Figure 1 Observation of cell growth after being treated with FB1 in different concentrations (bar=100 μm) |
细胞活力检测结果见图 2,与对照组相比,低质量浓度(2.5、5 μg·mL-1)的FB1短时间(6、12 h)处理对HUVEC的细胞活力没有显著性影响(P>0.05),其他各处理组的细胞活力均显著(P<0.05) 或极显著降低(P<0.01)。随着FB1药物质量浓度增加、时间增长,FB1对HUVEC形态和活力的影响增强,呈时间和剂量依赖性作用。
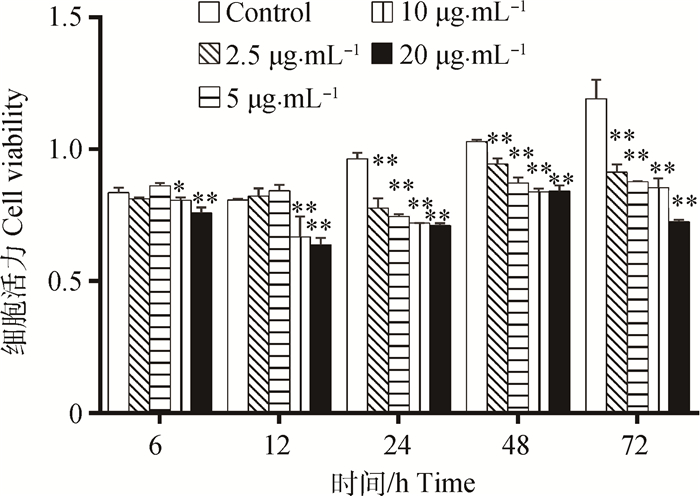
|
*.P < 0.05, **.P < 0.01 图 2 FB1对HUVEC细胞活力的影响 Figure 2 Effect of HUVEC cell viability in stimulation of FB1 with different concentrations at various times |
FB1对HUVEC细胞周期的影响结果如图 3、4所示。与对照组相比,FB1处理24 h试验组G0/G1期细胞所占比例:5 μg·mL-1组显著上升(P<0.05),10 μg·mL-1组显著上升(P<0.05),20 μg·mL-1组极显著上升(P<0.01);FB1处理48 h试验组G0/G1期细胞所占比例:5 μg·mL-1组显著上升(P<0.05),10 μg·mL-1组极显著上升(P<0.01),20 μg·mL-1组极显著上升(P<0.01);而FB1处理24、48 h试验组S期及G2/M期细胞所占比例均呈下降趋势。提示FB1处理后HUVEC从G0/G1期到S期发生了抑制,并且随着药物质量浓度增加、作用时间增长,抑制作用增强。
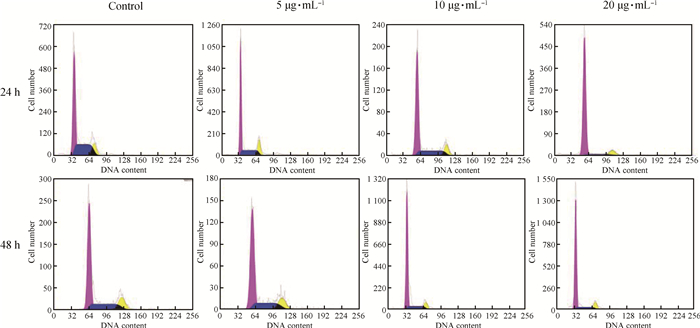
|
图 3 FB1对HUVEC细胞周期的影响 Figure 3 Effect of the FB1 on the cycle of HUVEC cells by flow cytometry instrument |
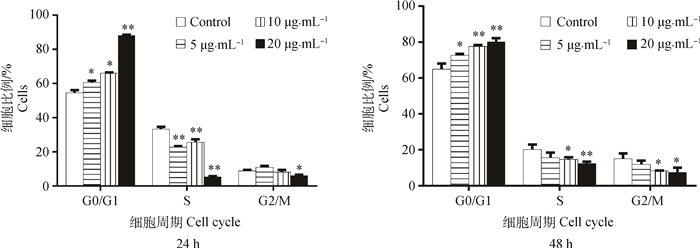
|
*.P < 0.05, **. P < 0.01 图 4 FB1对HUVEC细胞周期的检测结果 Figure 4 Results of cell cycle on HUVEC cells in stimulation of FB1 by flow cytometry instrument |
FB1对HUVEC中活性氧水平的影响结果如图 5。由图 5可见,与对照组相比,FB1组绿色荧光逐渐增强,细胞核呈浓缩、致密浓染。结果表明,FB1促使HUVEC内ROS积累增加,对细胞产生毒害作用。
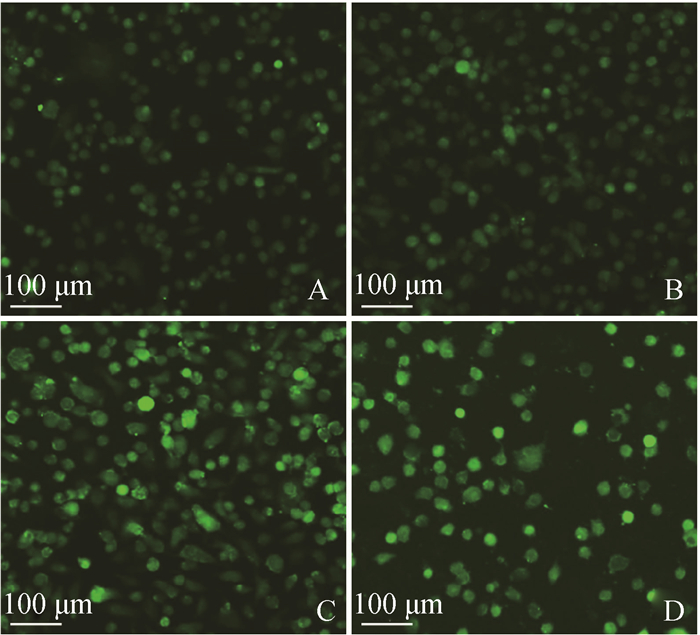
|
A.对照组48 h;B. 5 μg·mL-1组48 h;C. 10 μg·mL-1组48 h;D. 20 μg·mL-1组48 h A. Control group, 48 h; B. 5 μg·mL-1, 48 h; C. 10 μg·mL-1, 48 h; D. 20 μg·mL-1, 48 h 图 5 细胞内ROS水平变化的荧光检测 Figure 5 The detection of intracellular ROS variation by fluorescence |
FB1对HUVEC线粒体膜电位的影响结果见图 6、7。由图 6可见,与对照组相比,FB1处理组红色荧光逐渐减弱,绿色荧光逐渐增强,说明线粒体膜电位逐渐降低(编者注:作者因经费问题未彩印)。由图 7可见,流式结果显示,细胞内的线粒体膜电位逐渐降低,与荧光染色结果相符合。
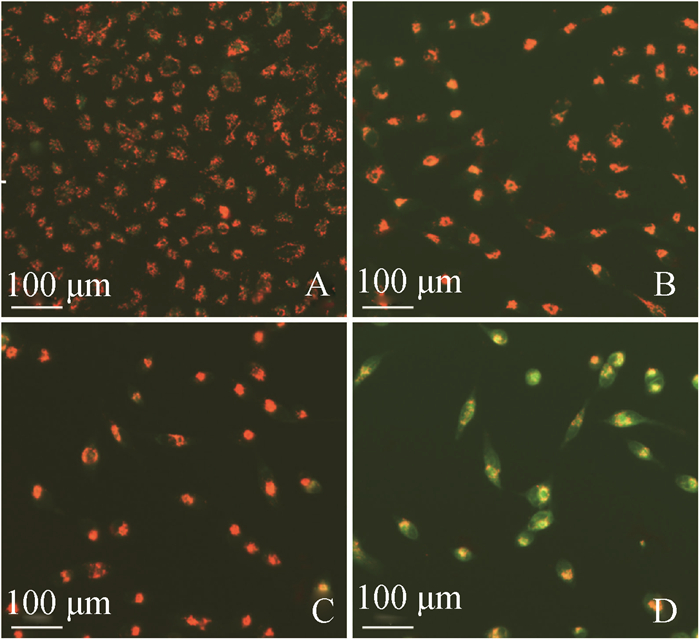
|
A.对照组48 h;B. 5 μg·mL-1组48 h;C. 10 μg·mL-1组48 h;D. 20 μg·mL-1组48 h A. Control group, 48 h; B. 5 μg·mL-1, 48 h; C. 10 μg·mL-1, 48 h; D. 20 μg·mL-1, 48 h 图 6 线粒体膜电位荧光染色结果 Figure 6 The fluorescence staining of mitochondrial membrane potential |
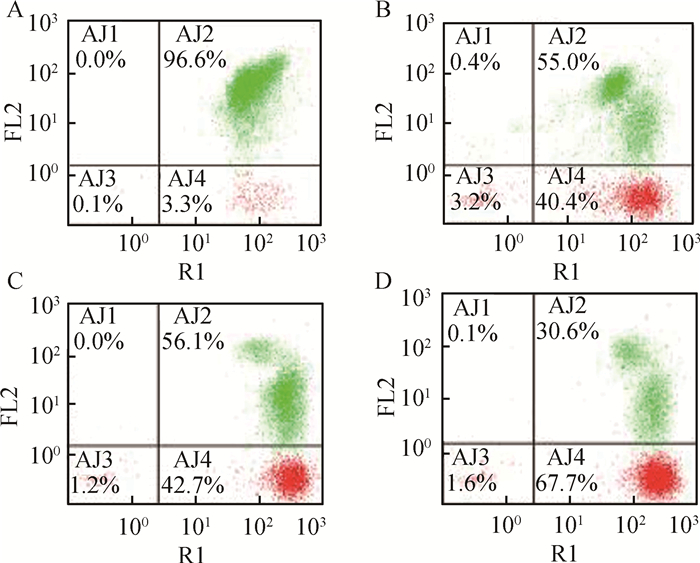
|
A.对照组48 h;B. 5 μg·mL-1组48 h;C. 10 μg·mL-1组48 h;D. 20 μg·mL-1组48 h A. Control group, 48 h; B. 5 μg·mL-1, 48 h; C. 10 μg·mL-1, 48 h; D. 20 μg·mL-1, 48 h 图 7 线粒体膜电位流式细胞仪检测 Figure 7 The mitochondrial membrane potential detected by flow cytometry instrument |
FB1对HUVEC Hoechst33342染色效果的影响结果见图 8。由图 8可见,与对照组相比,FB1组细胞的细胞核蓝色荧光明显增强,并且细胞核有不同程度的浓缩及致密浓染,这表明FB1诱导了HUVEC凋亡。
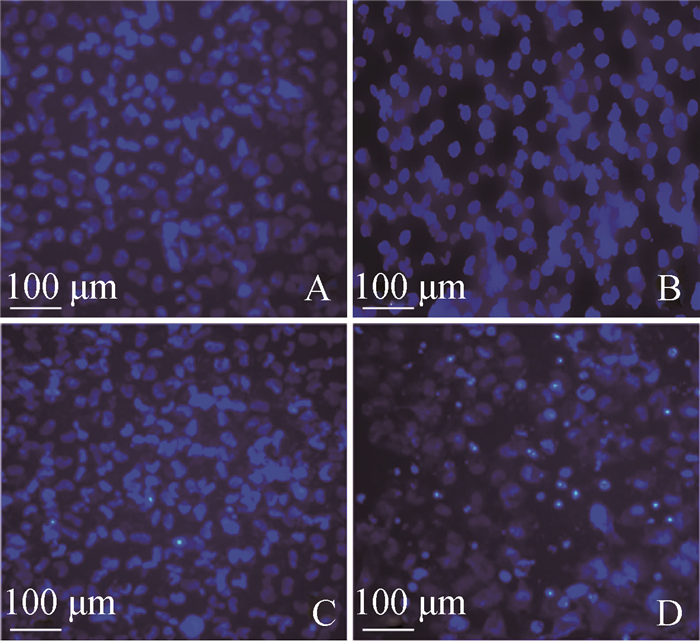
|
A.对照组48 h;B. 5 μg·mL-1组48 h;C. 10 μg·mL-1组48 h;D. 20 μg·mL-1组48 h A. Control group, 48 h; B. 5 μg·mL-1, 48 h; C. 10 μg·mL-1, 48 h; D. 20 μg·mL-1, 48 h 图 8 Hoechst33342染色结果 Figure 8 Results of Hoechst33342 dye staining |
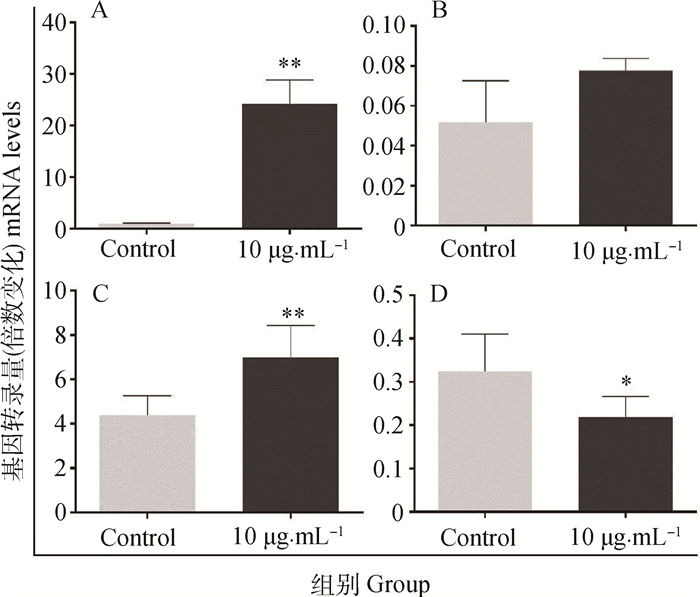
FB1对HUVEC细胞凋亡相关基因转录的影响结果见图 9。由图 9可见,与对照组相比,用FB1(10 μg·mL-1)处理HUVEC 48 h后,Caspase-9表达水平升高近30倍,差异极显著(P < 0.01);Caspase-3转录水平升高,但差异不显著(P>0.05);Bax转录水平上升1.5倍,差异极显著(P<0.01);Bcl-2转录水平明显降低,差异显著(P<0.05)。
|
A. Caspase-9;B. Caspase-3;C. Bax;D. Bcl-2 图 9 FB1对HUVEC凋亡相关基因转录的影响 Figure 9 Effect of FB1 incubation on apoptosis-related gene transcription in HUVEC |
伏马毒素广泛存在于自然界中,是粮食、饲料的主要污染源之一。人或畜禽通过饮食将伏马毒素摄入体内,会产生神经毒性,免疫毒性,细胞毒性,甚至致癌性,严重危害人类和畜禽健康,降低动物生产性能,带来不可估量的经济损失。伏马毒素进入机体后,上皮细胞比其他组织更早、更容易受到毒素的侵害[16]。HUVEC是一种典型的细胞模型,常用来研究内皮细胞的功能和变化。有研究报道,FB1能够诱导草地贪夜蛾sf9细胞、鼠神经瘤细胞肿胀、空泡化、黏附力下降[17-18],诱导人肠道上皮细胞、神经胶质瘤细胞发生G2/M期细胞周期阻滞[19-20]。通过形态学观察、细胞活力检测,本研究发现,FB1能够显著降低HUVEC细胞活力,抑制细胞的生长,导致细胞形态的畸变, 并呈时间和剂量依赖性。流式细胞术检测细胞周期,结果显示,FB1通过阻碍DNA的合成,将HUVEC细胞周期阻滞在G0/G1期,抑制HUVEC增殖,并存在时间和剂量依赖性。这可能是FB1抑制HUVEC增殖的重要因素之一。与人肠道上皮细胞等的差异可能是由于细胞类型不同所致。
J. J. Pestka等[21]报道,FB1能诱导氧化应激,阻止核糖体蛋白质合成。对人结肠腺癌细胞、大鼠肝细胞、大鼠脾单核细胞的研究表明,FB1诱导的细胞氧化应激与活性氧有关[22-23]。线粒体对细胞的氧化应激特别敏感,过剩的活性氧能诱导线粒体损伤[24-25]。细胞受到外部的损伤或者接收到外界压力应激信号时,线粒体途径最先作出反应[26-28]。本研究通过荧光标记法,发现FB1能够引起细胞内活性氧显著积累。通过荧光显微法和流式细胞术检测,结果显示,FB1能够降低HUVEC的线粒体膜电位,引起线粒体损伤,诱导细胞凋亡。与此一致,张荷(H. Zhang)等[17]研究发现FB1同样可引起昆虫细胞的线粒体膜电位超极化,使线粒体功能发生障碍。这一结果也表明FB1对多种类型细胞具有毒性作用。
细胞凋亡蛋白酶家族在感应、转换、放大细胞内的凋亡信号过程中发挥着重要作用。Caspase-3是这个家族里的一个关键基因,由Caspase-9激活[29]。本研究发现FB1能通过线粒体调控途径诱导HUVEC凋亡(图 7、8)。通过荧光定量PCR检测细胞凋亡相关基因Caspase-3、Caspase-9、Bax、Bcl-2的转录情况,从基因水平上证实了FB1对HUVEC的促凋亡作用。血管组织在机体内广泛分布,血管内皮细胞的正常增殖被抑制将导致人畜机体的心血管系统机能异常,继发一系列不良反应,危害人类和畜禽健康。本试验结果也从一定程度上解释了人畜因伏马毒素中毒而引起的肝、肾毒性,心血管毒性,免疫神经毒性等广泛毒性现象。研究结果为深入探讨伏马毒素的毒性作用机制、与人畜疾病的确切关系及相关疾病治疗奠定基础。
4 结论FB1在一定浓度范围内能使HUVEC形态发生改变,降低细胞活力,将细胞周期阻滞在G0/G1期,抑制HUVEC增殖。此外,FB1能升高HUVEC内活性氧水平,发生氧化应激,并通过降低线粒体膜电位引起线粒体损伤,诱导HUVEC凋亡。FB1诱导HUVEC毒性作用的具体分子机制还有待进一步研究。
| [1] | ESPOSITO F, FASANO E, SCOGNAMIGLIO G, et al. Exposure assessment to fumonisins B1, B2 and B3 through consumption of gluten-free foodstuffs intended for people affected by celiac disease[J]. Food Chem Toxicol, 2016, 97: 395–401. DOI: 10.1016/j.fct.2016.10.013 |
| [2] | WAŚKIEWICZ A, BESZTERDA M, GOLŃSKI P. Occurrence of fumonisins in food-an interdisciplinary approach to the problem[J]. Food Control, 2012, 26(2): 491–499. DOI: 10.1016/j.foodcont.2012.02.007 |
| [3] | BORDINI J G, ONO M A, GARCIA G T, et al. Impact of industrial dry-milling on fumonisin redistribution in non-transgenic corn in Brazil[J]. Food Chem, 2017, 220: 438–443. DOI: 10.1016/j.foodchem.2016.10.028 |
| [4] | GELINEAU-VAN WAES J, VOSS K A, STEVENS V L, et al. Maternal fumonisin exposure as a risk factor for neural tube defects[J]. Adv Food Nutr Res, 2009, 56: 145–181. DOI: 10.1016/S1043-4526(08)00605-0 |
| [5] | ISLAMI F, KAMANGAR F, NASROLLAHZADEH D, et al. Oesophageal cancer in Golestan Province, a high-incidence area in northern Iran-a review[J]. Eur J Cancer, 2009, 45(18): 3156–3165. DOI: 10.1016/j.ejca.2009.09.018 |
| [6] | ALIZADEH A M, ROSHANDEL G, ROUDBARMOHAMMADI S, et al. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran[J]. Asian Pac J Cancer Prev, 2012, 13(6): 2625–2628. DOI: 10.7314/APJCP.2012.13.6.2625 |
| [7] |
邱茂锋, 刘秀梅, 王玉华, 等. 某食管癌高发区人群伏马菌素摄入量及尿二氢神经鞘氨醇/神经鞘氨醇比值的调查[J]. 卫生研究, 2001, 30(6): 365–367.
QIU M F, LIU X M, WANG Y H, et al. Survey on the fumonisins intake and the urinary Sa/So ratio of people suffered from a high incidence of esophageal cancer[J]. Journal of Hygiene Research, 2001, 30(6): 365–367. (in Chinese) |
| [8] | DUTTON M F. Fumonisins, mycotoxins of increasing importance:their nature and their effects[J]. Pharmacol Ther, 1996, 70(2): 137–161. DOI: 10.1016/0163-7258(96)00006-X |
| [9] | BONDY G S, SUZUKI C A M, FERNIE S M, et al. Toxicity of fumonisin B1 to B6C3F1 mice:a 14-day gavage study[J]. Food Chem Toxicol, 1997, 35(10-11): 981–989. DOI: 10.1016/S0278-6915(97)87267-5 |
| [10] | DOMBRINK-KURTZMAN M A. Fumonisin and beauvericin induce apoptosis in turkey peripheral blood lymphocytes[J]. Mycopathologia, 2003, 156(4): 357–364. DOI: 10.1023/B:MYCO.0000003607.69016.d2 |
| [11] | GBORE F A. Growth performance and puberty attainment in growing pigs fed dietary fumonisin B1[J]. J Anim Physiol Anim Nutr, 2009, 93(6): 761–767. DOI: 10.1111/jpn.2009.93.issue-6 |
| [12] | COLLINS T F X, SPRANDO R L, BLACK T N, et al. Effects of Fumonisin B1 in Pregnant Rats. Part 2[J]. Food Chem Toxicol, 1998, 36(8): 673–685. |
| [13] | GALVANO F, RUSSO A, CARDILE V, et al. DNA damage in human fibroblasts exposed to fumonisin B1[J]. Food Chem Toxicol, 2002, 40(1): 25–31. DOI: 10.1016/S0278-6915(01)00083-7 |
| [14] | RIEDEL S, ABEL S, BURGER H M, et al. Differential modulation of the lipid metabolism as a model for cellular resistance to fumonisin B1-induced cytotoxic effects in vitro[J]. Prostaglandins Leukot Essent Fatty Acids, 2016, 109: 39–51. DOI: 10.1016/j.plefa.2016.04.006 |
| [15] | TOLLESON W H, MELCHIOR W J Jr, MORRIS S M, et al. Apoptotic and anti-proliferative effects of fumonisin B1 in human keratinocytes, fibroblasts, esophageal epithelial cells and hepatoma cells[J]. Carcinogenesis, 1996, 17(2): 239–249. DOI: 10.1093/carcin/17.2.239 |
| [16] | MARESCA M, YAHI N, YOUNèS-SAKR L, et al. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium:Stimulation of interleukin-8 secretion, potentiation of interleukin-1β effect and increase in the transepithelial passage of commensal bacteria[J]. Toxicol Appl Pharmacol, 2008, 228(1): 84–92. DOI: 10.1016/j.taap.2007.11.013 |
| [17] | ZHANG H, ZHANG L Y, DIAO X, et al. Toxicity of the mycotoxin fumonisin B1 on the insect Sf9 cell line[J]. Toxicon, 2017, 129: 20–27. DOI: 10.1016/j.toxicon.2017.01.018 |
| [18] | OSUCHOWSKI M F, SHARMA R P. Fumonisin B1 induces necrotic cell death in BV-2 cells and murine cultured astrocytes and is antiproliferative in BV-2 cells while N2A cells and primary cortical neurons are resistant[J]. NeuroToxicology, 2005, 26(6): 981–992. DOI: 10.1016/j.neuro.2005.05.001 |
| [19] | BONDY G S, BARKER M G, LOMBAERT G A, et al. A comparison of clinical, histopathological and cell-cycle markers in rats receiving the fungal toxins fumonisin B1 or fumonisin B2 by intraperitoneal injection[J]. Food Chem Toxicol, 2000, 38(10): 873–886. DOI: 10.1016/S0278-6915(00)00084-3 |
| [20] | MOBIO T A, ANANE R, BAUDRIMONT I, et al. Epigenetic properties of fumonisin B1:cell cycle arrest and DNA base modification in C6 glioma cells[J]. Toxicol Appl Pharmacol, 2000, 164(1): 91–96. DOI: 10.1006/taap.2000.8893 |
| [21] | PESTKA J J, ZHOU H R, MOON Y, et al. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes:unraveling a paradox[J]. Toxicol Lett, 2004, 153(1): 61–73. DOI: 10.1016/j.toxlet.2004.04.023 |
| [22] | MECA G, FERNÁNDEZ-FRANZÓN M, RITIENI A, et al. Formation of fumonisin B1-glucose reaction product, in vitro cytotoxicity, and lipid peroxidation on kidney cells[J]. J Agric Food Chem, 2010, 58(2): 1359–1365. DOI: 10.1021/jf9028255 |
| [23] | MARY V S, THEUMER M G, ARIAS S L, et al. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells[J]. Toxicology, 2012, 302(2-3): 299–307. DOI: 10.1016/j.tox.2012.08.012 |
| [24] | DOMIJAN A M, ABRAMOV A Y. Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis-implication to mechanism of cell toxicity[J]. Int J Biochem Cell Biol, 2011, 43(6): 897–904. DOI: 10.1016/j.biocel.2011.03.003 |
| [25] | POERSCH A B, TROMBETTA F, BRAGA A C M, et al. Involvement of oxidative stress in subacute toxicity induced by fumonisin B1 in broiler chicks[J]. Vet Microbiol, 2014, 174(1-2): 180–185. DOI: 10.1016/j.vetmic.2014.08.020 |
| [26] | HOU Q, CYMBALYUK E, HSU S C, et al. Apoptosis modulatory activities of transiently expressed Bcl-2:roles in cytochrome c release and Bax regulation[J]. Apoptosis, 2003, 8(6): 617–629. DOI: 10.1023/A:1026187526113 |
| [27] | WON S J, CHUNG K S, KI Y S, et al. CWJ-081, a novel 3-arylisoquinoline derivative, induces apoptosis in human leukemia HL-60 cells partially involves reactive oxygen species through c-Jun NH2-terminal kinase pathway[J]. Bioorg Med Chem Lett, 2010, 20(22): 6447–6451. DOI: 10.1016/j.bmcl.2010.09.078 |
| [28] | ZHAO Y Y, SHEN X, CHAO X, et al. Ergosta-4, 6, 8(14), 22-tetraen-3-one induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells[J]. Biochim Biophys Acta, 2011, 1810(4): 384–390. DOI: 10.1016/j.bbagen.2010.12.005 |
| [29] | ALNEMRI E S. Mammalian cell death proteases:a family of highly conserved aspartate specific cysteine proteases[J]. J Cell Biochem, 1997, 64(1): 33–42. DOI: 10.1002/(ISSN)1097-4644 |



