2. 四川农业大学动物医学院, 成都 611130
2. College of Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130, China
黄曲霉毒素(aflatoxins,AFs)主要是由黄曲霉、寄生曲霉和寄生曲霉属真菌产生的次生代谢产物,在农产品生产及加工过程中均可产生[1]。在已知黄曲霉毒素(B1、B2、G1、G2、M1)中,AFB1的毒性和致癌性最强[2]。AFB1除了抑制动物生长性能,明显导致肝、肾等器官损伤外[3],还具有极强的免疫毒性作用[4]。大量研究表明,AFB1导致动物对营养物质的吸收率降低[5],降低动物免疫器官的脏器指数[6-7],并抑制巨噬细胞的活性[8]。雏鸡和鸭对其易感性较强[9]。现有研究表明,AFB1降低雏鸡血清中抗体滴度水平[10],诱导免疫细胞凋亡[11],降低外周血、胸腺、脾和肠黏膜上皮CD3+、CD3+CD4+和CD3+CD8+ T淋巴细胞比例并增加胸腺CD4+CD8+ T淋巴细胞比例[12],降低血清中TNF-α、IL-17和IFN-γ等细胞因子水平[13]。
家禽的胸腺功能紊乱将引起胸腺免疫缺陷和自体免疫性疾病[14]。AFB1对雏鸡胸腺的免疫抑制作用可能与免疫细胞过度凋亡有关[6],且AFB1可能通过启动线粒体通路和死亡受体通路诱导雏鸡胸腺细胞凋亡[11],但是其具体的网络调控机制尚不明确。FADD/TRADD等信号分子可通过介导自体反应性胸腺细胞的凋亡来抑制其增殖[15],从而减少自体免疫应答。笔者以雏鸡为模型,通过检测雏鸡胸腺脏器指数、T淋巴细胞亚群比例、凋亡细胞的比例以及凋亡相关基因的mRNA转录情况,探究AFB1诱导胸腺细胞凋亡的死亡受体通路活化机制,并探讨胸腺细胞凋亡与T淋巴细胞亚群改变的关系。
1 材料与方法 1.1 实验动物与日粮组成90只1日龄科宝肉鸡健雏(购于成都市温江区正大有限公司)被随机分为两个组:对照组(0 mg·kg-1 AFB1)和AFB1组(0.6 mg·kg-1 AFB1),试验期为21 d。根据国际研究委员会标准(NRC, 1994) 和中国肉鸡饲养标准(NY/T33-2004) 配制基础日粮,其构成成分及其营养水平见表 1。AFB1日粮配制方法如下:将27 mg AFB1充分溶解于30 mL甲醇后,加至100 g基础日粮中混匀。然后将混有甲醇的日粮置于98 ℉(37 ℃)烘箱中,每隔2 h称重一次,待前后两次质量差小于2 g时,认为甲醇已挥发殆尽,再将其逐级混匀至44.9 kg基础日粮中以制备45 kg AFB1日粮。在基础日粮中添加等量甲醇,按照相同的方法制备45 kg对照日粮。经高效液相色谱仪(HPLC,Milford,MA,USA)检测,AFB1日粮中AFB1的含量为0.601 mg·kg-1,对照日粮中AFB1的含量低于检测限,即少于0.001 mg·kg-1。
|
|
表 1 试验日粮组成及营养水平表 Table 1 Ingredients and nutrition level of the experimental diet |
于7、14和21日龄时,随机抽取每组5只雏鸡剖杀,观察胸腺的眼观变化。去除胸腺周围的结缔组织后用电子天平称其净重并记录,根据如下公式计算脏器指数。
脏器指数=脏器净重(g)/空腹体重(kg)
1.3 胸腺T淋巴细胞亚群检测于7、14和21日龄时,随机抽取每组5只雏鸡,颈静脉放血处死,迅速剥离胸腺并剔除周围结缔组织,置于4 ℃磷酸盐缓冲液(PBS, pH=7.2~7.6) 备用。胸腺组织经眼科剪剪碎制成组织匀浆,300目尼龙网过滤后,500~800 r·min-1低速离心,弃上清液,PBS洗两次后重悬细胞,制成浓度为1×106·mL-1的单细胞悬液。取100 μL上述单细胞悬液于流式管中,分别加入鼠抗鸡CD3-SPRD (8200-13,Southern Biotech,美国),鼠抗鸡CD4-FITC(8210-02,Southern Biotech,美国),鼠抗鸡CD8-RPE(8220-09,Southern Biotech,美国)单克隆抗体各一头份,4 ℃避光染色15 min。用2 mL PBS洗一次,离心后弃上清,以450 μL PBS重悬细胞后上流式细胞仪(FACSCalibur,BD,美国)检测。通过Flowjo6.0软件分析获得CD3+、CD3+CD4+、CD3+CD8+和CD4+CD8+ T淋巴细胞百分率。
1.4 胸腺细胞凋亡检测 1.4.1 Annexin V-FITC/PI双染色法检测胸腺细胞凋亡率取上述单细胞悬液100 μL于流式管中,用Annexin V-FITC/PI试剂盒(51-66211E,BD Pharmingen,美国)染色。染色步骤根据试剂盒使用说明书进行。简言之:加入5 μL Annexin-V-FITC和5 μL PI,轻微振荡混匀,25 ℃避光静置15 min;加入400 μL结合缓冲液,振荡混匀,流式细胞仪检测,Flowjo6.0软件分析凋亡率。
1.4.2 荧光定量PCR检测凋亡相关基因的变化于7、14和21日龄时,每组随机选择5只雏鸡,剖杀后立即分离胸腺并冻存于液氮。用研钵将胸腺组织磨成粉末状,装入无RNA酶EP管中,-80 ℃保存备用。
自NCBI GenBank获得相关基因,经NCBI Blast比对设计引物,并由上海生物工程有限公司合成(表 2)。
|
|
表 2 荧光定量PCR引物 Table 2 The primers for fluorogenic quantitative PCR detection |
总RNA的提取采用TriPure法,根据试剂盒使用说明书(11667165001,Roche,德国)进行;经核酸蛋白仪检测总RNA浓度及A260 nm/A280 nm值(控制在1.8~2.0);总RNA经罗氏反转录试剂盒(04897030001,Roche,德国)反转录获得cDNA,-80 ℃保存备用。
用罗氏SYBR Green荧光染料(04913914001,Roche,德国)进行荧光标记,并通过Bio-Rad荧光定量PCR仪(Bio-Rad,CFX96 touch,美国)进行检测;反应采用20 μL体系进行:10 μL荧光染料,0.5 μL正向引物,0.5 μL反向引物,1 μL cDNA模板,8 μL灭菌蒸馏水。反应条件设定:95 ℃预变性2 min;95 ℃变性10 s,50~60 ℃退火30 s,72 ℃延伸30 s,进行40个循环;经95 ℃ 1 min,55 ℃ 1 min,55~98 ℃(86个循环)获取熔解曲线。
1.5 数据分析数据均采用SPSS 20.0统计软件进行数据统计,结果以平均数(x)±标准差(s)表示。并采用独立样本T检验方法分析组间差异的显著性,P < 0.05表示差异显著,P < 0.01表示差异极显著。
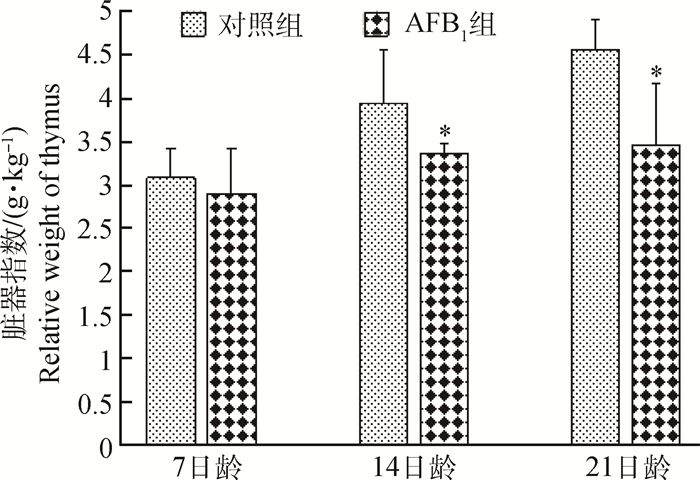
2 结果 2.1 胸腺的脏器指数变化雏鸡胸腺的脏器指数变化情况如图 1所示。7日龄时,AFB1组胸腺脏器指数无明显变化(P < 0.05);14和21日龄时,AFB1组胸腺脏器指数显著低于对照组(P < 0.05)。
|
*表示与对照组比较差异显著(P < 0.05) * represents difference (P < 0.05) compared with that in the control group 图 1 雏鸡胸腺的脏器指数变化 Figure 1 Changes of the relative weight of thymus |
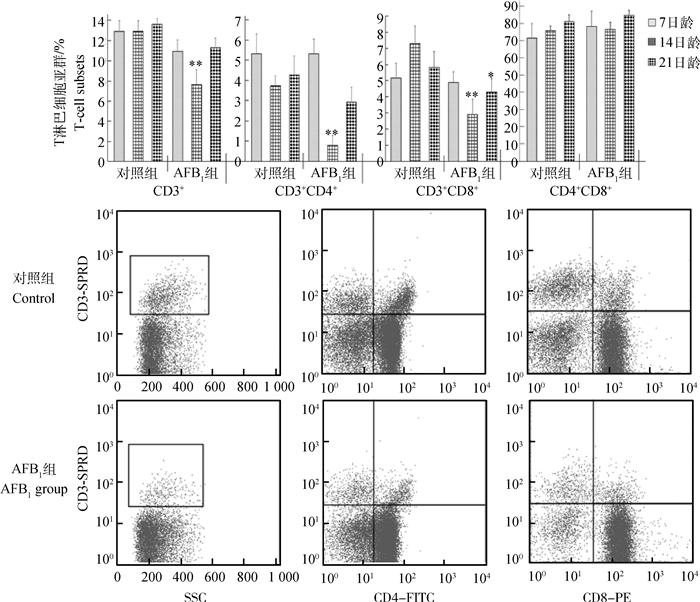
雏鸡胸腺T淋巴细胞亚群变化情况如图 2所示。与对照组比较,AFB1组CD3+和CD3+CD4+ T淋巴细胞百分率在14日龄时极显著降低(P < 0.01),在7和21日龄时变化不明显(P>0.05);CD3+CD8+ T淋巴细胞百分率在7日龄时变化不明显(P>0.05),在14和21日龄时显著或极显著降低(P < 0.05或P < 0.01);CD4+CD8+细胞百分率变化不明显(P>0.05)。
|
直方图中,*表示与对照组比较差异显著(P < 0.05),**表示差异极显著(P < 0.01),下图同。流式象限图则直观地表明,AFB1组雏鸡胸腺CD3+、CD3+CD4+和CD3+CD8+T淋巴细胞比例较对照组降低 When compared with that in the control group, * represents difference (P < 0.05), and ** represents significant difference (P < 0.01) in the histograms, the same as below. Quadrantal diagram by the flow cytometry also intuitively show that the percentages of CD3+, CD3+CD4+, and CD3+CD8+ T lymphocyts were decrease compared with those in the control group 图 2 雏鸡胸腺T淋巴细胞亚群变化 Figure 2 Changes of the percentage of thymic T-cell subsets in chickens |
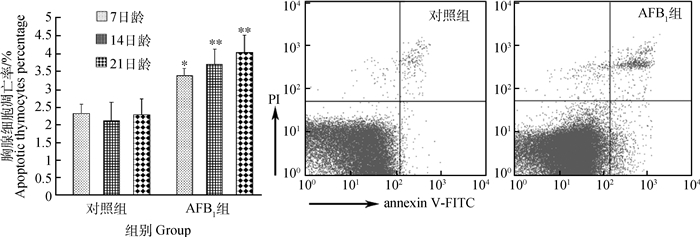
雏鸡胸腺细胞凋亡率变化情况如图 3所示。通过Annexin V-FITC/PI双染色法及流式细胞术检测显示,7日龄时AFB1组胸腺细胞凋亡率明显高于对照组(P < 0.05);14和21日龄时,AFB1组胸腺细胞凋亡率极显著高于对照组(P < 0.01)。流式象限图中,右上和右下象限分别代表晚期凋亡细胞和早期凋亡细胞,本试验中所统计的凋亡细胞为早期和晚期凋亡细胞之和。流式图直观地显示出,AFB1组的凋亡细胞百分比与对照组比较有明显增多。
|
图 3 雏鸡胸腺细胞凋亡率变化 Figure 3 Changes of the percentage of apoptotic thymocytes in chickens |
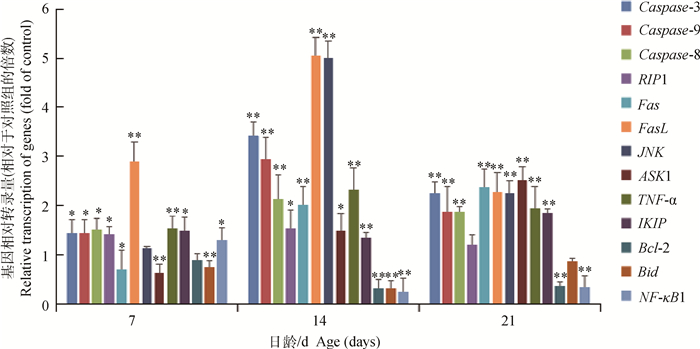
与对照组比较,AFB1组雏鸡胸腺细胞Caspase-8、Caspase-3、Caspase-9、TNF-α、FasL、JNK和IKIP的mRNA转录量于7、14和21日龄时显著或极显著升高(P < 0.01或P < 0.05);Fas和ASK1的转录量虽在7日龄时降低(P < 0.05或P < 0.01),但在14和21日龄时显著或极显著升高(P < 0.01或P < 0.05);RIP1的mRNA相对转录量在7和14日龄时显著升高(P < 0.05)。与对照组相比,AFB1组胸腺细胞Bid、Bcl-2和NF-κB1的mRNA相对转录量在7、14和21日龄时显著或极显著降低(P < 0.01或P < 0.05)。
|
图 4 AFB1组雏鸡胸腺细胞凋亡调控基因的相对转录量(2-ΔΔCT法) Figure 4 The relative transcription of apoptosis regulating genes in thymus from the broilers in the AFB1 group compared with those in the control group (using the 2-ΔΔCT method) |
胸腺是雏鸡重要的中枢免疫器官之一,是T淋巴细胞增殖分化的场所,与机体的细胞免疫功能密切相关。已有研究表明,AFB1可引起雏鸡[16]和鸭[17]胸腺脏器指数降低。本试验也观察到AFB1组雏鸡胸腺的脏器指数在14和21日龄时显著降低,表明日粮中添加0.6 mg·kg-1 AFB1可能对雏鸡胸腺的发育有一定抑制作用。本试验中也观察到胸腺细胞凋亡率升高及成熟T淋巴细胞百分率下降,因此,AFB1日粮导致雏鸡胸腺脏器指数降低的机制可能与胸腺细胞过度凋亡和T淋巴细胞分化成熟受阻有关。
3.2 AFB1日粮对胸腺T淋巴细胞亚群的影响胸腺细胞由骨髓造血祖细胞产生,通过血液循环到达胸腺内分化成熟,将经历以下几个阶段[18]:① 前体T淋巴细胞增殖分化为双阴性(CD4-CD8-)T淋巴细胞;② 由CD4-CD8- T淋巴细胞分化为双阳性(CD4+CD8+)T淋巴细胞;③ 成熟单阳性T淋巴细胞形成,细胞膜表面出现CD3分子,产生CD3+CD4+CD8-和CD3+CD8+CD4-细胞;④ 进入外周循环。处于分化阶段的胸腺细胞对有害刺激较敏感[19-20]。与我们的前期研究结果[16]相似,本试验观察到0.6 mg·kg-1 AFB1组雏鸡胸腺CD3+、CD3+CD4+和CD3+CD8+ T淋巴细胞百分率显著降低,其原因可能与AFB1促进CD3+CD4+和CD3+CD8+胸腺细胞的凋亡有关。已有研究表明,不同凋亡相关因子活化可介导胸腺细胞分化成熟的不同阶段[21]。本试验及笔者的前期研究[11]显示,AFB1日粮可使雏鸡胸腺中Fas、Bak、Bax和FADD等促凋亡因子的mRNA过量转录,而抑凋亡因子NF-κB1的mRNA转录下调,这些凋亡调控因子的改变是否直接参与调控胸腺成熟T淋巴细胞百分率下降,尚有待进一步研究。
3.3 死亡受体通路基因活化与AFB1所致的胸腺细胞凋亡率上调有报道指出,AFB1可诱导大鼠[22]、雏鸡[6]和猪[23]的胸腺细胞凋亡上调。本试验观察到,摄食0.6 mg·kg-1AFB1导致雏鸡胸腺细胞凋亡率升高。细胞凋亡可能是导致雏鸡胸腺脏器指数降低和胸腺内成熟T淋巴细胞百分率降低的重要原因之一。我们课题组的前期研究表明,AFB1可能通过活化线粒体通路和死亡受体通路来介导雏鸡胸腺细胞过度凋亡[11],但就死亡受体通路而言,前期的试验仅检测了Fas、FasL和TRADD等启动因子,对其下游因子的变化情况尚不清楚。本研究采用定量PCR法检测相关基因的转录情况,探究死亡受体通路在AFB1所致的雏鸡胸腺细胞凋亡上调中的网络调控作用。
死亡受体通路是在死亡受体(Fas、TNF-α、TRALL和DR5等)作用下介导细胞凋亡的信号途径。综合本试验和笔者前期研究[11]的结果,AFB1主要通过以下三条死亡受体通路途径诱导胸腺细胞凋亡(图 5):① Fas-FasL-FADD-caspase-8-caspase-3途径。本试验中,AFB1组胸腺组织的Fas、FasL、FADD、Caspase-8和Caspase-3的mRNA转录量与对照组比较均有升高。在胸腺细胞和成熟T淋巴细胞内,Fas在FasL和FADD作用下,相继激活Caspase-8、Caspase-3和Caspase-7,直接促进细胞凋亡[24]。② 通过活化Fas-ASK1-JNK-Bcl-2途径,并在线粒体的介导下,启动下游调控因子Caspase-9和Caspase-3诱导细胞凋亡。本试验中,AFB1组胸腺的Fas、ASK1和JNK的mRNA转录量上调,而Bcl-2的转录量下调。有研究指出,Fas可与Daxx结合后,相继活化ASK1和JNK[25],促进Bcl-2磷酸化,导致Bcl-2表达量减少,继而引起线粒体损伤[26];在Bcl-2表达水平较低时,活化后的Caspase-8可将细胞质内Bid水解为tBid,相继激活Bax/Bak后,引起线粒体膜损伤并释放凋亡信号分子[27],增强Caspase-9和Caspase-3的活性,进而诱导细胞凋亡。Caspase-3又可催化Bid水解,进一步促进线粒体内凋亡分子的释放[27]。③ 通过TNF-α-RIP1-IKIP-NF-κB1-Caspase-3信号途径诱导胸腺细胞凋亡。研究指出,TNF-α诱导的细胞凋亡主要受NF-κB信号途径的调控[28]。TNF-α可通过上调RIP1的表达,诱导IKK活化[29];IKIP是IKK结合蛋白,可以靶向IKK负向调节NF-κB1的活性[30],减少对Caspase-3的抑制,促进细胞凋亡。本试验结果表明,AFB1可能通过TNF-α-RIP1-IKIP-NF-κB1途径,活化Caspase-3促进细胞凋亡。此外,促炎因子TNF-α可通过上调RIP的表达启动JNK信号通路,进而通过途径① 促进细胞凋亡[31]。也有研究表明NF-κB1可正向调节Bcl-2的活性发挥抗凋亡作用[32]。本试验结果显示,AFB1组雏鸡胸腺NF-κB1和Bcl-2的转录均下调,提示NF-κB1的下调可能负向调节Bcl-2的活性,致其抗凋亡作用减弱,进而诱导线粒体损伤并促进细胞凋亡。有研究表明,Fas和TNF-α可分别介导CD4+和CD8+成熟T淋巴细胞的凋亡[33]。本试验观察到摄食AFB1能明显降低雏鸡胸腺CD3+CD4+和CD3+CD8+ T淋巴细胞百分率,此过程可能受Fas和TNF-α的调控。
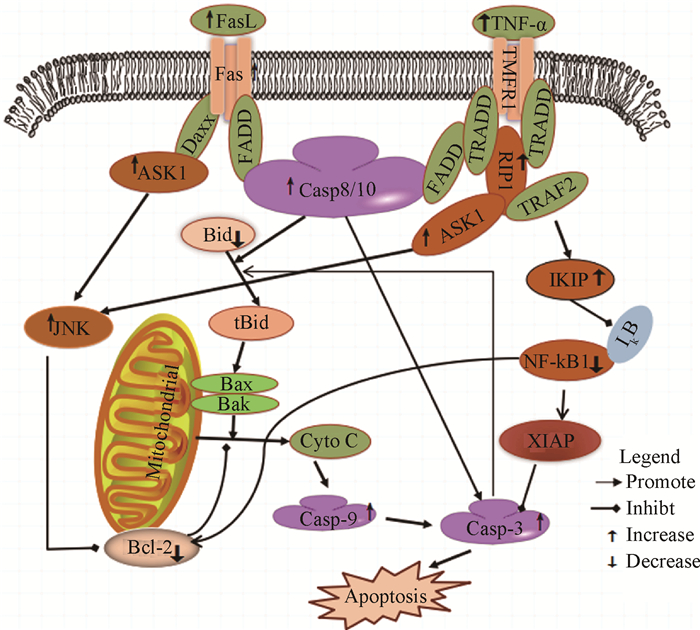
|
图 5 通过死亡受体通路诱导胸腺细胞凋亡的模拟示意 Figure 5 Schematic diagram of the proposed mechanisms of AFB1-induced thymocytes apoptosis through death receptor pathway |
摄食含0.6 mg·kg-1 AFB1的日粮可致雏鸡胸腺中的成熟T淋巴细胞百分率降低和胸腺细胞凋亡率升高。日粮AFB1导致雏鸡胸腺细胞过度凋亡的过程中,死亡受体通路的三条下游信号途径均有活化,即Fas-FasL-FADD-Caspase-8-Caspase-3途径、Fas-ASK1-JNK-Bcl-2途径和TNF-α-RIP1-IKIP-NF-κB1-Caspase-3途径。
| [1] | GOURAMA H, BULLERMAN L B. Aspergillus flavus and Aspergillus parasiticus:aflatoxigenic fungi of concern in foods and feeds:a review[J]. J Food Protect, 1995, 58(12): 1395–1404. DOI: 10.4315/0362-028X-58.12.1395 |
| [2] | SINGHAL K K, KAUR H. Aflatoxins in livestock and poultry:a review[J]. Indian J Anim Sci, 2005, 75(1): 113–120. |
| [3] | REDZWAN S M, ROSITA J, SOKHINI A M M, et al. Detection of serum AFB1-lysine adduct in Malaysia and its association with liver and kidney functions[J]. Int J Hyg Environ Health, 2014, 217(4-5): 443–451. DOI: 10.1016/j.ijheh.2013.08.007 |
| [4] | OSWALD I P, MARIN D E, BOUHET S, et al. Immunotoxicological risk of mycotoxins for domestic animals[J]. Food Addit Contam, 2005, 22(4): 354–360. DOI: 10.1080/02652030500058320 |
| [5] | HAN X Y, HUANG Q C, LI W F, et al. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels[J]. Livest Sci, 2008, 119(1-3): 216–220. DOI: 10.1016/j.livsci.2008.04.006 |
| [6] | PENG X, CHEN K J, CHEN J, et al. Aflatoxin B1 affects apoptosis and expression of Bax, Bcl-2, and Caspase-3 in thymus and bursa of fabricius in broiler chickens[J]. Environ Toxicol, 2016, 31(9): 1113–1120. DOI: 10.1002/tox.v31.9 |
| [7] |
于正强, 陈瑾, 彭西, 等. 黄曲霉毒素B1对雏鸡免疫器官影响的病理学观察[J]. 畜牧兽医学报, 2015, 46(8): 1447–1454.
YU Z Q, CHEN J, PENG X, et al. Effect of aflatoxin B1 on pathological changes of immune organs in broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(8): 1447–1454. (in Chinese) |
| [8] | PAUL S, JAKHAR R, BHARDWAJ M, et al. Glutathione-S-transferase omega 1(GSTO1-1) acts as mediator of signaling pathways involved in aflatoxin B1-induced apoptosis-autophagy crosstalk in macrophages[J]. Free Radical Biol Med, 2015, 89: 1218–1230. DOI: 10.1016/j.freeradbiomed.2015.11.006 |
| [9] | GHIMPETT, EANU O M, TOLESCU A, MILITARU M. Aflatoxin and ochratoxin contamination in poultry-a review[J]. Sci Works-Univ Agronom Sci Vet Med Bucharest Ser C, Vet Med, 2012, 58(3): 308–317. |
| [10] | TESSARI E N C, OLIVEIRA C A F, CARDOSO A L S P, et al. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks[J]. Br Poult Sci, 2006, 47(3): 357–364. DOI: 10.1080/00071660600756071 |
| [11] | PENG X, YU Z Q, LIANG N, et al. The mitochondrial and death receptor pathways involved in the thymocytes apoptosis induced by aflatoxin B1[J]. Oncotarget, 2016, 7(11): 12222–12234. DOI: 10.18632/oncotarget.v7i11 |
| [12] | JIANG M, PENG X, FANG J, et al. Effects of aflatoxin B1 on T-cell subsets and mRNA expression of cytokines in the intestine of broilers[J]. Int J Mol Sci, 2015, 16(4): 6945–6959. DOI: 10.3390/ijms16046945 |
| [13] | BAKHEET S A, ATTIA S M, ALWETAID M Y, et al. β-1, 3-Glucan reverses aflatoxin B1-mediated suppression of immune responses in mice[J]. Life Sci, 2016, 152: 1–13. DOI: 10.1016/j.lfs.2016.03.030 |
| [14] | GIRARD N. Thymic epithelial tumours:from basic principles to individualised treatment strategies[J]. Eur Respir Rev, 2013, 22(127): 75–87. DOI: 10.1183/09059180.00007312 |
| [15] | NEWTON K, HARRIS A W, BATH M L, et al. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes[J]. EMBO J, 1998, 17(3): 706–718. DOI: 10.1093/emboj/17.3.706 |
| [16] | CHEN K J, SHU G, PENG X, et al. Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1[J]. Food Chem Toxicol, 2013, 59: 446–454. DOI: 10.1016/j.fct.2013.06.032 |
| [17] | HE J, ZHANG K Y, CHEN D W, et al. Effects of vitamin E and selenium yeast on growth performance and immune function in ducks fed maize naturally contaminated with aflatoxin B1[J]. Livest Sci, 2013, 152(2-3): 200–207. DOI: 10.1016/j.livsci.2012.12.018 |
| [18] | CONTE D, LISTON P, WONG J W, et al. Thymocyte-targeted overexpression of xiap transgene disrupts T lymphoid apoptosis and maturation[J]. Proc Natl Acad Sci U S A, 2001, 98(9): 5049–5054. DOI: 10.1073/pnas.081547998 |
| [19] | DAVEY G M, SCHOBER S L, ENDRIZZI B T, et al. Preselection thymocytes are more sensitive to t cell receptor stimulation than mature T cells[J]. J Exp Med, 1998, 188(10): 1867–1874. DOI: 10.1084/jem.188.10.1867 |
| [20] | URBANI S, AMADEI B, FISICARO P, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses[J]. Hepatology, 2006, 44(1): 126–139. |
| [21] | NOSSAL G J V. Negative selection of lymphocytes[J]. Cell, 1994, 76(2): 229–239. DOI: 10.1016/0092-8674(94)90331-X |
| [22] | ABBÈS S, BEN SALAH-ABBÈS J, JEBALI R, et al. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress:possible protective role using lactic acid bacteria[J]. J Immunotoxicol, 2016, 13(1): 46–54. DOI: 10.3109/1547691X.2014.997905 |
| [23] | SURAI P F, DVORSKA J E. Effects of mycotoxins on antioxidant status and immunity[M]//DIAZ D E. The Mycotoxin Blue Book. Nottingham:Nottingham University Press, 2005:93-137. |
| [24] | KAUFMANN T, STRASSER A, JOST P J. Fas death receptor signalling:roles of Bid and XIAP[J]. Cell Death Differ, 2012, 19(1): 42–50. DOI: 10.1038/cdd.2011.121 |
| [25] | YANIV G, SHILKRUT M, LARISCH S, et al. Hydrogen peroxide predisposes neonatal rat ventricular myocytes to Fas-mediated apoptosis[J]. Biochem Biophys Res Commun, 2005, 336(3): 740–746. DOI: 10.1016/j.bbrc.2005.08.167 |
| [26] | MAUNDRELL K, ANTONSSON B, MAGNENAT E, et al. Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1[J]. J Biol Chem, 1997, 272(40): 25238–25242. DOI: 10.1074/jbc.272.40.25238 |
| [27] | SLEE E A, KEOGH S A, MARTIN S J. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3:a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release[J]. Cell Death Differ, 2000, 7(6): 556–565. DOI: 10.1038/sj.cdd.4400689 |
| [28] | ZHU G Z, WU C J, ZHAO Y G, et al. Optineurin Negatively Regulates TNFα-Induced NF-κB Activation by Competing with NEMO for Ubiquitinated RIP[J]. Curr Biol, 2007, 17(16): 1438–1443. DOI: 10.1016/j.cub.2007.07.041 |
| [29] | DEVIN A, COOK A, LIN Y, et al. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1:TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation[J]. Immunity, 2000, 12(4): 419–429. DOI: 10.1016/S1074-7613(00)80194-6 |
| [30] |
赵学英. IKIP通过靶向IKKβ负向调节NF-κB介导的炎性细胞因子的表达[D]. 济南: 山东大学, 2013.
ZHAO X Y. IκB kinase interacting protein negatively regulates production of proinflammatory cytokines by targeting IKKβ[D]. Jinan:Shandong University, 2013. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10422-1013218476.htm |
| [31] | JACKSON-BERNITSAS D G, ICHIKAWA H, TAKADA Y, et al. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-κB activation and proliferation in human head and neck squamous cell carcinoma[J]. Oncogene, 2007, 26(10): 1385–1397. DOI: 10.1038/sj.onc.1209945 |
| [32] | HECKMAN C A, MEHEW J W, BOXER L M. NF-κB activates Bcl-2 expression in t(14;18) lymphoma cells[J]. Oncogene, 2002, 21(24): 3898–3908. DOI: 10.1038/sj.onc.1205483 |
| [33] | ZHENG L X, FISHER G, MILLER R E, et al. Induction of apoptosis in mature T cells by tumour necrosis factor[J]. Nature, 1995, 377(6547): 348–351. DOI: 10.1038/377348a0 |



