2. 河北省深州市职业技术教育中心, 深州 053800
2. Shenzhou of Hebei Province Vocational and Technical Education Center, Shenzhou 053800, China
皮肤黑色素细胞负责合成黑素体中的黑色素以保护角质形成细胞免受紫外线(UV)辐射等有害因素刺激,根据UV光谱波长分为3组:UVA(320~400 nm)、UVB(280~320 nm)和UVC(200~280 nm)。在这些UV波长中,UVB对黑色素细胞具有黑色素生成的特性[1-3]。UVB辐射可以强烈诱导MITF和酪氨酸酶的表达[4]。MITF作为黑色素细胞发育、功能和生存的主要调控因子[5],其可以被SOX10和PAX3蛋白调节[6]。MITF基因包含9种不同的启动子[7],而在黑色素细胞中只有M启动子(MITF-M)可以选择性表达[8]。MITF-M启动子直接反式激活Wnt/β-catenin促进神经嵴衍生的色素细胞分化[9],此外,小眼畸形转录因子(MITF)表达和功能的调节与BRAF/MEK/ERK/MAP-激酶(MAPK)途径相关[10],并且ERK可以激活MITF的磷酸化[11]。黑色素瘤细胞中α-MSH刺激也可以激活MC1R-MITF信号途径[12]。PAK4在体内介导UVB诱导黑色素生成,活化的PAK4通过两种不同的信号传导途径促进黑色素生成:CREB/MITF/酪氨酸酶和β-catenin/MITF信号传导途径[4]。MITF直接调控相关色素基因的转录,包括黑色素生成调控酶(TYR、TYRP1、TYRP2),黑素体蛋白GPNMB[13]以及转运蛋白SLC45A2(MATP/AIM-1)[14-15]等。其中与黑色素生成3种调控酶以及转运蛋白的功能已被人所熟知,而黑素体蛋白GPNMB的功能还未被完全理解。GPNMB表达依赖于MITF的激活促进[16],P.Zhang等[17]则认为GPNMB表达以MITF独立的方式进行。GPNMB和PMEL在黑素体生物发生中起重要作用的高度同源蛋白质[18-19]。OA1与黑素体的数量、大小、能动性和成熟相关,且与GPNMB表达无关[20]。巨噬细胞相关的GPNMB经由CD44通过ERK和AKT信号通路介导间充质干细胞生存、增殖和迁移[21]。最新研究表明,GPNMB通过离子通道中Na+/K+-ATP(NKA)酶激活PI3K/Akt和MEK/ERK信号通路[22]。而离子转运蛋白SLC45A2参与黑素体蛋白TYR、TYRP1、TYRP2在细胞内精确地加工和运输,其作为质子依赖性转运蛋白与植物蔗糖质子同向转运表现出结构同源性,并且可能指导黑素体蛋白和其他物质转运到黑素体[23-24]。目前对GPNMB关于毛色的研究未见报道,对色素生成机理性的研究鲜有阐明以及UVB对小鼠黑色素细胞中GPNMB的影响少有提及。本研究通过不同生物学方法探索GPNMB在色素调控中的作用。
1 材料与方法 1.1 试剂与材料RIPA裂解液(碧云天公司)、TRIZOL(Invitrogen公司)、SYBR® Premix Ex TapTMⅡ(TaKaRa公司)、MELM(2 201)(Sciencell)、0.25%胰酶(索莱宝公司)、GPNMB兔抗多克隆抗体(三鹰公司)、X-tremeGENE HP DNA Transfection Reagent(上海宇博生物公司)、C57BL/6J品系小鼠、C57BL/6J小鼠黑色素细胞5代、慢病毒载体pLV.Des3d.P/Puro以及pcDNA3.1(+)(本实验室提供)。
随机选取C57BL/6J品系出生12 d的黑、棕、灰小鼠各3只,背部剪毛,每只小鼠各取3块皮肤组织,1块在Bouin’s液中固定,制作石蜡切片和免疫组织化学染色。其余2块组织用于总蛋白质及总RNA的提取。
1.2 免疫组织化学将石蜡组织切片经梯度酒精复水,滴加3% H2O2室温静置(10 min),PBS摇洗3次(每次3 min);滴加5%山羊血清室温封闭10 min,弃掉切片上的血清并滴加1:100倍GPNMB兔抗多克隆抗体,阴性对照滴加抗体稀释液;4 ℃过夜;次日37 ℃复温30 min,用PBS摇洗3次(每次3 min);滴加1:150倍HRP-山羊抗兔二抗工作液,放入37 ℃温箱孵育10 min,用PBS摇洗3次(每次3 min);滴加DAB显色2~5 min,PBS摇洗3次(每次3 min),苏木素轻度复染、梯度酒精脱水、二甲苯透明,使用中性树胶进行封片、显微镜下观察。
1.3 蛋白提取和Western blot按照碧云天裂解液试剂盒提取不同毛色小鼠皮肤组织和每组黑色素细胞的总蛋白,每个样品总蛋白上样量为200 μg,待SDS-PAGE电泳结束后转移至NC膜;NC膜经5%脱脂奶粉室温封闭1 h;加入GPNMB、MC1R、β-catenin均是(1:1 000) 和β-actin(1:2 000) 一抗,4 ℃过夜孵育;次日复温30 min。NC膜用TBST摇洗3次(每次10 min);加入HRP标记的二抗(1:10 000),37 ℃恒温水平摇床孵育NC膜1 h。NC膜用TBST摇洗6次(每次5 min),使用ECL试剂盒显色后,进行胶片曝光,扫描获取目的图像,用Image-ProPlus 6.0软件对目的基因和β-actin免疫印迹结果进行分析。测定目的条带面积和灰度值,进行半定量分析。
1.4 RNA提取和qRT-PCRTrizol法提取不同毛色小鼠皮肤和每组细胞的总RNA,测定其浓度后反转录。在NCBI上检索小鼠的GPNMB、MC1R、β-catenin、SLC45A2序列,利用Premier 5.0软件设计PCR扩增引物,送北京华大基因公司合成。引物序列和产物长度见表 1。
|
|
表 1 目的基因引物序列及PCR扩增产物 Table 1 Sequence of primer and product size of PCR |
按照SYBR® Premix Ex TapTM Ⅱ试剂盒说明书进行荧光定量PCR,通过2-△△CT法计算目的mRNA相对表达量变化。
1.5 小鼠GPNMB、SLC45A2克隆载体和真核表达载体构建克隆载体构建:首先,Enzyme Solution(Solution I)5 μL;目的基因PCR胶回收产物4 μL;T-Vector pMD19(Simple)1 μL。16 ℃连接14~16 h;随后转化、涂板;次日进行蓝白斑筛选,37 ℃水平摇床200 r·min-1培养12~16 h,提取质粒后,送华大基因公司测序,确定克隆载体是否构建成功。
真核表达载体构建:克隆载体双酶切,回收的目的基因产物5 μL;表达载体产物4 μL;T4 DNA Ligase 1 μL。混匀,PCR仪中,16 ℃过夜连接;随后转化、涂板;次日挑菌后,37 ℃水平摇床200 r·min-1培养12~16 h,提取质粒后,送华大基因公司测序,确定真核表达载体是否构建成功。
1.6 黑色素细胞复苏、转染小鼠黑色素细胞复苏、培养24 h,待细胞达到75%~80%融合时进行基因转染。首先,将200 μL(无血清双抗)培养基稀释7.5 μL转染试剂,混匀后室温静置5 min。其次,将5.0 μg DNA组加入混合液中,混匀后,室温静置15 min。最后,滴加到含有1 mL新鲜培养基的培养板中,6孔·板-1补至2 mL,37 ℃培养56 h。
1.7 UVB辐射条件和剂量将飞利浦公司生产的UVB台式紫外线灯(9 w),调整合适的高度后,使用UVX-340紫外线辐照计测量辐照度,UVB剂量(mJ·cm-2)=UVB辐照度(μw·cm-2)×时间(s)。每次照射前,弃掉培养基减小误差,加入适量PBS后,分别选用0、50、100、200、300 mJ·cm-2进行照射。加入新鲜培养基继续培养,24 h后,采用四甲基偶氮噻唑蓝比色法(MTT法)测定细胞活性。
黑色素细胞复苏后,取对数生长期的正常细胞,0.25%胰酶+0.02% EDTA消化,用细胞计数仪计数,并将细胞浓度调节为1×104个·mL-1,接种于96孔板,置于5% CO2培养箱中,培养24 h,弃掉培养基,加入50 μL PBS后,分别用本试验选用的条件进行UVB辐射,再加入新鲜的黑色素培养基,放入培养箱中继续培养24 h。弃掉培养基,每孔加入5 mg·mL-1的MTT溶液20 μL,放置培养箱孵育4 h,每孔加二甲基亚砜(DMSO)150 μL,使其充分溶解。在酶标仪上测各孔490 nm吸光度值,并绘制黑色素细胞的生长曲线,从中选取细胞增殖活性最高的紫外照射剂量进行后续试验。
1.8 黑色素细胞UVB照射培养将黑色素细胞取对数生长期且为正常细胞,胰酶消化后用细胞计数仪计数,并将细胞浓度调节为1×106个·mL-1,接种于60 cm2细胞培养皿中(每皿2 mL),置于5% CO2培养箱中培养24 h,弃掉培养基,加入2 mL PBS后,用上述最佳的剂量进行照射,加入新鲜的黑色素培养基,置于培养箱中继续培养,并按照设置的分组收集细胞。用0、50、100、200、300 mJ·cm-2剂量UVB分别对细胞照射0、1、3、5 d,当细胞形态出现明显变化时收集细胞,并对GPNMB和MC1R的mRNA表达量进行检测。
1.9 数据分析所有数据均用SPSS19.0软件进行单因素方差分析,所有结果均用“平均值±标准误(Mean±SE)”表示,所有柱形图均用GraphPad PrismTM(GraphPad Software, Inc.California, USA)处理。
2 结果 2.1 GPNMB在不同毛色小鼠皮肤中表达免疫组织化学结果显示,GPNMB在不同毛色小鼠皮肤的毛基质、内外毛根鞘等处均呈现出棕黄色的阳性反应,并且在内外毛根鞘部位着色程度略深(图 1A)。Western blot结果表明:在不同毛色小鼠皮肤的总蛋白可以与GPNMB抗体发生免疫学阳性反应(图 1B1),通过对蛋白条带分析可知,GPNMB在黑色和棕色小鼠皮肤中蛋白水平分别是灰色小鼠的1.92倍(P<0.05) 和1.81倍(P<0.05)(图 1B2)。qRT-PCR结果显示:GPNMB在黑色和棕色小鼠皮肤中mRNA相对表达量分别是灰色小鼠的6.25倍(P<0.01) 和3.61倍(P<0.01)(图 1C1)。1%琼脂糖凝胶电泳后显示,目的条带清晰且单一。条带大小为GPNMB(215 bp)(图 1C2)。切胶后送华大基因公司进行测序,序列比对正确,表明GPNMB在不同毛色小鼠皮肤中可正常表达。由此可以看出,GPNMB在不同毛色小鼠皮肤中表达量存在差异性,说明GPNMB通过某种调控机制参与毛色的形成。
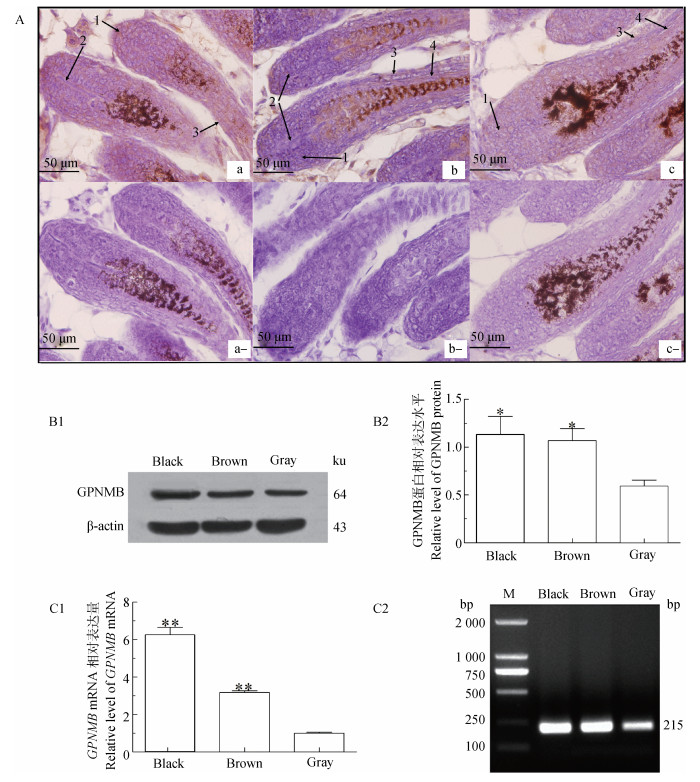
|
**.P < 0.01; *.P < 0.05,下同。A.GPNMB在不同毛色小鼠毛囊的免疫定位;a、b、c.黑、棕、灰小鼠皮肤GPNMB阳性组;a-、b-、c-.黑、棕、灰小鼠皮肤GPNMB阴性对照组;1.毛基质;2.毛乳头;3.外根鞘;4.内根鞘;B1.GPNMB在不同毛色小鼠皮肤的蛋白印迹;B2和C1.GPNMB在不同毛色小鼠皮肤中蛋白和mRNA相对水平;C2.不同毛色小鼠皮肤GPNMB PCR产物;M.DL 2000 DNA marker **.P < 0.01; *.P < 0.05; The same as below. A.Immunohistochemistry results of GPNMB in different hair color mouse skin. a, b, c.Black, brown, gray skin GPNMB positive group; a-, b-, c-.Black, brown, gray skin GPNMB negative control group; 1.Hair follicle matrix; 2.Dermal papilla; 3.Outside root sheath; 4.Inside root sheath; B1.Immunoblot results of GPNMB protein in different colors of mouse skin; B2, C1.Relative expression level of GPNMB protein and mRNA in different skin colors of mouse skin; C2.GPNMB PCR products of different skin colors of mouse; M.DL 2000 DNA marker 图 1 不同毛色小鼠皮肤中GPNMB表达量分析 Figure 1 Analysis of the expression of GPNMB in different skin colors of mouse |
过表达GPNMB结果显示:与空载体组(Vector)对比,试验组(Vector-GPNMB)的MC1R蛋白升高1.98倍(P < 0.01);mRNA升高8.86倍(P < 0.01)(图 2)。说明过表达GPNMB可以使MC1R的表达水平升高。
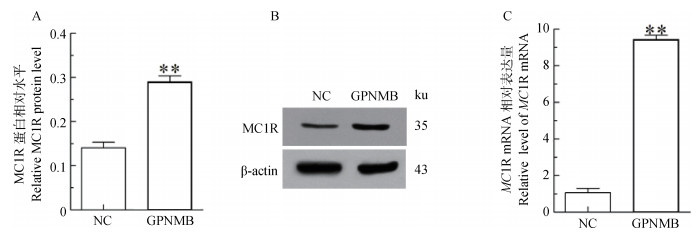
|
A.MC1R蛋白相对水平;B.MC1R蛋白印迹;C.MC1R mRNA相对表达量。NC.转染空载体的黑色素细胞(Vector);GPNMB.转染GPNMB的黑色素细胞(Vector-GPNMB) A.Relative expression level of MC1R protein; B.Immunoblot results of MC1R protein; C. Relative expression level of MC1R mRNA; NC.The melanocytes transfected with empty vector(Vector); GPNMB.The melanocytes transfected with GPNMB(Vector-GPNMB) 图 2 转染GPNMB后黑色素细胞中MC1R的表达 Figure 2 Analysis of MC1R expression in melanocytes transfected by GPNMB |
过表达GPNMB结果表明(图 3):与空载组(Vector)对比,试验组(Vector-GPNMB)的β-catenin蛋白升高1.45倍(P < 0.05);mRNA升高1.38倍(P < 0.05)。说明过表达GPNMB可以使β-catenin的表达水平升高。
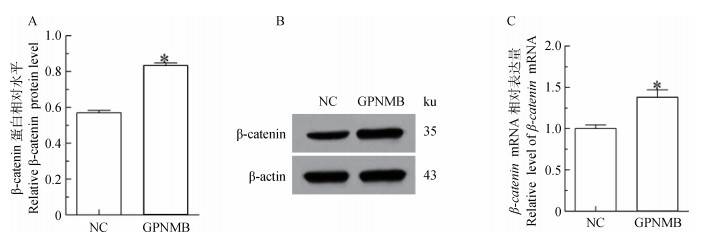
|
A.β-catenin蛋白相对水平;B.β-catenin蛋白印迹;C.β-catenin mRNA相对表达量;NC.转染空载体的黑色素细胞(Vector);GPNMB.转染GPNMB的黑色素细胞(Vector-GPNMB) A.Relative expression level of β-catenin protein; B.Immunoblot results of β-catenin protein; C.Relative expression level of β-catenin mRNA; NC.The melanocytes transfected with empty vector(Vector). GPNMB.The melanocytes transfected with GPNMB(Vector-GPNMB) 图 3 转染GPNMB后黑色素细胞中β-catenin的表达 Figure 3 Analysis of β-catenin expression in melanocytes transfected by GPNMB |
过表达GPNMB结果显示:与空载体组(Vector)对比,试验组(Vector-GPNMB)的SLC45A2 mRNA升高6.19倍(P < 0.01)(图 4A)。过表达SLC45A2结果显示:与空载体组(Vector)对比,试验组(Vector-SLC45A2) 的GPNMB蛋白升高1.73倍(P < 0.01);mRNA升高2.17倍(P < 0.01)(图 4B~D)。表明GPNMB与SLC45A2之间存在一定的关系,两者可能通过调控黑素体的成熟而影响色素的生成。由于经费原因,没有购买SLC45A2抗体,所以没有检测SLC45A2蛋白表达。
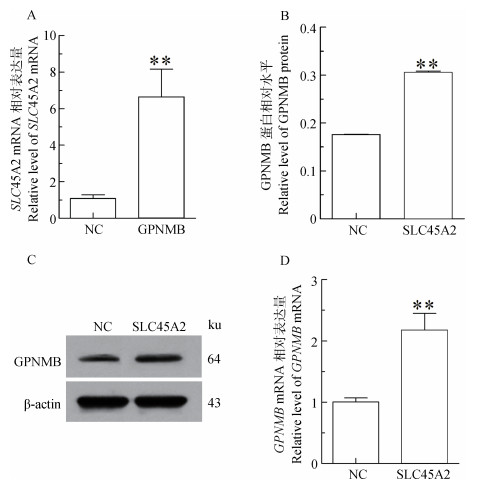
|
A.SLC45A2 mRNA相对表达量;B.GPNMB蛋白相对水平;C.GPNMB蛋白印迹;D.GPNMB mRNA相对表达量;NC.转染空载体的黑色素细胞(Vector);GPNMB.转染GPNMB的黑色素细胞(Vector-GFP);SLC45A2.转染SLC45A2的黑色素细胞(Vector-SLC45A2) A.Relative expression level of SLC45A2 mRNA; B.Relative expression level of GPNMB protein; C.Immunoblot results of GPNMB protein; D. Relative expression level of GPNMB mRNA; NC.The melanocytes transfected with empty vector(Vector). GPNMB.The melanocytes transfected with GPNMB(Vector-GPNMB). SLC45A2.The melanocytes transfected with SLC45A2 (Vector-SLC45A2) 图 4 转染GPNMB、SLC45A2后黑色素细胞中色素相关基因的表达 Figure 4 Analysis of pigment-related genes expression in melanocytes transfected by GPNMB, SLC45A2 |
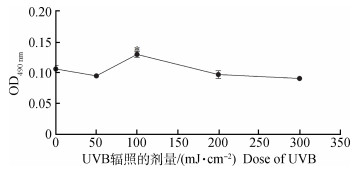
通过不同剂量(0、50、100、200、300 mJ·cm-2)的UVB对黑色素细胞进行照射,观察24 h时细胞增殖活性,使细胞增殖活性最高的剂量为100 mJ·cm-2(P<0.05),而其他条件对细胞的增殖活性无影响(图 5)。本研究选取100 mJ·cm-2作为最佳的照射剂量。
|
图 5 不同剂量UVB照射黑色素细胞24 h后细胞活性测定 Figure 5 Measurement of cell viability after different doses of UVB irradiation treated with melanocytes for 24 h |
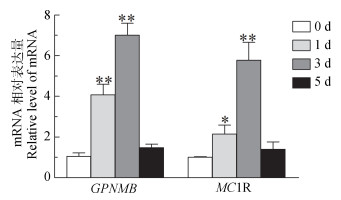
UVB在100 mJ·cm-2剂量对黑色素细胞分别照射0、1、3、5 d收集细胞对GPNMB mRNA表达量进行检测发现,GPNMB在1、3、5 d的mRNA相对表达量分别是0 d的3.89倍(P<0.01)、6.70倍(P<0.01)、1.41倍;MC1R在1、3、5 d的mRNA相对表达量分别是0 d的2.15倍(P<0.05)、5.77倍(P<0.01)、1.40倍。由此可以看出,UVB照射对GPNMB和MC1R产生相似的变化,进一步说明GPNMB对小鼠黑色素细胞有影响,其可能是黑色素生成必不可少的条件(图 6)。
|
图 6 UVB照射黑色素细胞后对GPNMB和MC1R的影响 Figure 6 Effects of UVB irradiation on GPNMB and MC1R in melanocytes |
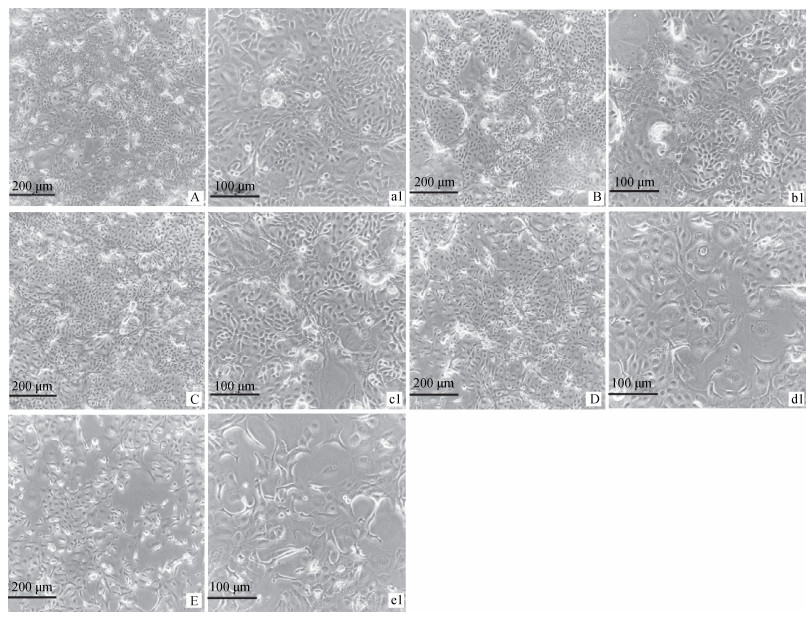
分别选用0、50、100、200、300 mJ·cm-2剂量对黑色素细胞进行照射。当连续照射5 d时,UVB在200和300 mJ·cm-2剂量细胞形态发生明显变化。与UVB在0、50、100 mJ·cm-2剂量照射相比黑色素细胞的体积和树突变大(图 7)。
|
A、a1.正常黑色素细胞;B、b1. 50 mJ·cm-2 UVB;C、c1. 100 mJ·cm-2 UVB;D、d1.200 mJ·cm-2UVB;E、e1.300 mJ·cm-2 UVB A, a1.Normal melanocytes; B, b1. 50 mJ·cm-2 UVB; C, c1. 100 mJ·cm-2 UVB; D, d1.200 mJ·cm-2 UVB; E, e1.300 mJ·cm-2 UVB 图 7 不同剂量UVB辐射对黑色素细胞形态的影响 Figure 7 Effects of different doses of UVB radiation on the morphology of melanocytes |
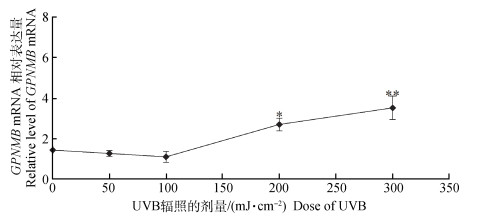
分别对选用0、50、100、200、300 mJ·cm-2剂量连续辐射5 d的黑色素细胞,并且对GPNMB mRNA表达量进行检测,结果表明,GPNMB mRNA表达量在200(P<0.05) 和300 mJ·cm-2(P<0.01) 剂量最高(图 8)。说明UVB对小鼠黑色素细胞中的GPNMB有影响。
|
图 8 UVB辐射对黑色素细胞GPNMB的影响 Figure 8 Effects of UVB radiation on GPNMB in melanocytes |
个体和群体之间毛发色素形成的多样性源自褐黑素(红色/黄色)和真黑素(黑色/棕色)的比例差异。黑色素合成过程发生于黑色素细胞内溶酶体相关的囊泡(称为黑素体),这些囊泡被转移到树突顶部以及角质化细胞的周围使皮肤和头发的色素沉着[25]。通常黑素体螯合在黑色素合成过程中产生的细胞毒性中间体,而GPNMB和TYRP1都是跨膜黑素体蛋白,其突变导致细胞毒性中间体从黑素体渗漏,从而导致虹膜细胞的变性和色素的分散[26]。GPNMB参与小鼠青光眼和人黑素瘤的发育[19],在发育过程中GPNMB类似于MITF,TYRP2和PMEL的模式表达[27]。此外,GPNMB以RGD依赖的方式黏附PAM212角化细胞,其可能参与黑素细胞的发育,黑色素合成和黑素瘤的产生[19, 28-29]。最新研究表明,GPNMB在不同毛囊时期小鼠皮肤的表达量变化与TYR、TYRP2、OA1、PMEL等存在一致性[30]。本试验探究GPNMB在不同毛色小鼠皮肤表达量是否存在差异性,结果表明,GPNMB在不同毛色小鼠皮肤表达量不同,且在黑色小鼠皮肤的表达量>棕色>灰色,结合上述GPNMB功能和表达,说明GPNMB对毛色的形成有影响,本试验结果为进一步研究其对黑色素的生成以及如何参与毛色形成奠定了基础。
在色素调控通路中MITF不仅是许多色素沉着基因的调控因子,而且是Wnt/β-catenin信号通路[31],MC1R/α-MSH信号通路[32-33]以及SCF/C-KIT信号通路[11, 21, 34-36]的调控枢纽。前两种通路可以升高MITF表达,而第3种通路可以通过ERK的作用降低MITF的表达。这些通路中都是经由MITF调控TYR、TYRP1和TYRP2的转录而影响黑色素的生成[37]。过表达GPNMB后,TYR、TYRP1、TYRP2、OA1、PMEL表达量均升高,而MITF表达量下降[38]。由此推测GPNMB过表达可以影响MITF的上游途径,进而调控MITF的变化。MITF表达的调控机制是一个错综复杂的系统,并且MITF包含位于N-末端的强转录激活结构域(TAD)以及C-末端的弱TAD[39],所以至少有4个转录因子参与黑素细胞中MITF基因的反式激活,其中包括转录因子PAX3、SOX10、Wnt/β-catenin通路效应LEF-1以及cAMP途径效应物cAMP反应蛋白结合元件(CREB)[40]。此外,乳腺癌抑制因子候选物-1(BCSC-1) 可通过与SOX10结合来下调MITF[41],以及MITF表达也可被转录因子GL12和转化生长因子b抑制[42]等。因此,反馈回路应该是控制这个途径的活动。鉴于此基础,本试验以小鼠黑色素细胞为研究对象,继续利用细胞转染技术,过表达GPNMB进一步检测MC1R和β-catenin表达量的变化。结果显示MC1R和β-catenin表达量均升高,结合上述信号通路中MITF为调控枢纽的作用可知,GPNMB过表达MCIR和β-catenin表达量升高而导致MITF表达量升高,而ERK信号传导途径致使MITF表达量降低。最终结果GPNMB过表达可能使MITF表达量升高的程度小于其减低的程度。此外,本研究结果表明,过表达GPNMB可以使SLC45A2表达量升高,而SLC45A2具有控制酪氨酸酶的活性、黑素体的运输以及维持黑色素小体pH的功能[14, 43-44]。相关资料显示,敲除MATP显著降低黑素体pH,导致酪氨酸酶活性降低,最终使黑色素的含量减少[45],以及通过降低SLC45A2的效率,降低黑素体的pH,对酪氨酸酶的活性产生抑制[44]。所以GPNMB过表达可以使黑色素含量增加,这与先前研究的结果相符[38]。
UV感应以P53依赖的方式引起的DNA损伤发生在角质形成细胞中,随后导致α-MSH肽的分泌[46],而α-MSH结合胞质膜上的MC1R时,可以调节色素沉着相关基因的变化[33]。相关研究表明,UVB照射可以增加GPNMB的表达,可能UVB有助于黑素体的成熟[17, 47]。本试验通过UVB对小鼠黑色素细胞进行处理,结果显示,UVB对小鼠黑色素细胞中GPNMB有一定的影响,并且与MC1R表达趋势一致。由此可知,UVB可能通过激活MC1R/α-MSH信号通路进而调控色素沉着相关基因的变化影响黑素体的成熟,最终引起GPNMB表达量发生改变。
4 结论GPNMB在不同毛色小鼠皮肤存在差异表达,并且通过调控MITF的上下游进而影响色素的生成。此外,UVB可以改变GPNMB的表达量,可能通过某种通路影响黑素体的成熟来调节其和黑色素的变化。
| [1] | CICHOREK M, WACHULSKA M, STASIEWICZ A, et al. Skin melanocytes:biology and development[J]. Postepy Dermatol Alergol, 2013, 30(1): 30–41. |
| [2] | CHOI W, MIYAMURA Y, WOLBER R, et al. Regulation of human skin pigmentation in situ by repetitive UV exposure:molecular characterization of responses to UVA and/or UVB[J]. J Invest Dermatol, 2010, 130(6): 1685–1696. DOI: 10.1038/jid.2010.5 |
| [3] | MIYAMURA Y, COELHO S G, SCHLENZ K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin[J]. Pigment Cell Melanoma Res, 2011, 24(1): 136–147. DOI: 10.1111/j.1755-148X.2010.00764.x |
| [4] | YUN C Y, YOU S T, KIM J H, et al. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways[J]. J Invest Dermatol, 2015, 135(5): 1385–1394. DOI: 10.1038/jid.2014.548 |
| [5] | LEVY C, KHALED M, FISHER D E. MITF:master regulator of melanocyte development and melanoma oncogene[J]. Trends Mol Med, 2006, 12(9): 406–414. DOI: 10.1016/j.molmed.2006.07.008 |
| [6] | STEINGRIMSSON E, COPELAND N G, JENKINS N A. Melanocytes and the microphthalmia transcription factor network[J]. Annu Rev Genet, 2004, 38: 365–411. DOI: 10.1146/annurev.genet.38.072902.092717 |
| [7] | HERSHEY C L, FISHER D E. Genomic analysis of the Microphthalmia locus and identification of the MITF-J/Mitf-J isoform[J]. Gene, 2005, 347(1): 73–82. DOI: 10.1016/j.gene.2004.12.002 |
| [8] | FUSE N, YASUMOTO K I, SUZUKI H, et al. Identification of a melanocyte-type promoter of the microphthalmia-associated transcription factor gene[J]. Biochem Biophys Res Commun, 1996, 219(3): 702–707. DOI: 10.1006/bbrc.1996.0298 |
| [9] | SCHMIDT C, PATEL K. Wnts and the neural crest[J]. Anat Embryol Berl, 2005, 209(5): 349–355. DOI: 10.1007/s00429-005-0459-9 |
| [10] | YASUMOTO K I, YOKOYAMA K, TAKAHASHI K, et al. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes[J]. J Biol Chem, 1997, 272(1): 503–509. DOI: 10.1074/jbc.272.1.503 |
| [11] | KIM D S, HWANG E S, LEE J E, et al. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation[J]. J Cell Sci, 2003, 116(Pt 9): 1699–1706. |
| [12] | HAYASHI E, HACHIYA K, KOJO S, et al. α-MSH stimulation contributes to TGF-β1 production via MC1R-MITF signaling pathway in melanoma cell[J]. Inflammat Regenerat, 2015, 35(5): 244–254. DOI: 10.2492/inflammregen.35.244 |
| [13] | WETERMAN M A J, AJUBI N, VAN DINTER I M R, et al. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts[J]. Int J Cancer, 1995, 60(1): 73–81. DOI: 10.1002/(ISSN)1097-0215 |
| [14] | DU J Y, FISHER D E. Identification of Aim-1 as the underwhite mouse mutant and its transcriptional regulation by MITF[J]. J Biol Chem, 2002, 277(1): 402–406. DOI: 10.1074/jbc.M110229200 |
| [15] | VITAVSKA O, WIECZOREK H. The SLC45 gene family of putative sugar transporters[J]. Mol Aspects Med, 2013, 34(2-3): 655–660. DOI: 10.1016/j.mam.2012.05.014 |
| [16] | GUTKNECHT M, GEIGER J, JOAS S, et al. The transcription factor MITF is a critical regulator of GPNMB expression in dendritic cells[J]. Cell Commun Signal, 2016, 13: 19. |
| [17] | ZHANG P, LIU W, ZHU C S, et al. Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion[J]. PLoS One, 2012, 7(8): e42955. DOI: 10.1371/journal.pone.0042955 |
| [18] | THEOS A C, WATT B, HARPER D C, et al. The PKD domain distinguishes the trafficking and amyloidogenic properties of the pigment cell protein PMEL and its homologue GPNMB[J]. Pigment Cell Melanoma Res, 2013, 26(4): 470–486. DOI: 10.1111/pcmr.2013.26.issue-4 |
| [19] | TOMIHARI M, HWANG S H, CHUNG J S, et al. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion[J]. Exp Dermatol, 2009, 18(7): 586–595. DOI: 10.1111/exd.2009.18.issue-7 |
| [20] | CHEN T Z, WANG H D, LIU Y, et al. Ocular Albinism Type 1 regulates melanogenesis in mouse melanocytes[J]. Int J Mol Sci, 2016, 17(10): 1596. DOI: 10.3390/ijms17101596 |
| [21] | WU B, SONDAG G R, MALCUIT C, et al. Macrophage-associated osteoactivin/GPNMB mediates mesenchymal stem cell survival, proliferation, and migration via a CD44-dependent mechanism[J]. J Cell Biochem, 2016, 117(7): 1511–1521. DOI: 10.1002/jcb.25394 |
| [22] | ONO Y, TSURUMA K, TAKATA M, et al. Glycoprotein nonmetastatic melanoma protein B extracellular fragment shows neuroprotective effects and activates the PI3K/Akt and MEK/ERK pathways via the Na+/K+-ATPase[J]. Sci Rep, 2016, 6: 23241. DOI: 10.1038/srep23241 |
| [23] | COSTIN G E, VALENCIA J C, VIEIRA W D, et al. Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (uw) mutation. A model for oculocutaneous albinism (OCA) type 4[J]. J Cell Sci, 2003, 116(pt15): 3203–3212. |
| [24] | CULLINANE A R, VILBOUX T, O'BRIEN K, et al. Homozygosity mapping and whole-exome sequencing to detect SLC45A2 and G6PC3 mutations in a single patient with oculocutaneous albinism and neutropenia[J]. J Invest Dermatol, 2011, 131(10): 2017–2025. DOI: 10.1038/jid.2011.157 |
| [25] | HEARING V J. Biogenesis of pigment granules:a sensitive way to regulate melanocyte function[J]. J Dermatol Sci, 2005, 37(1): 3–14. DOI: 10.1016/j.jdermsci.2004.08.014 |
| [26] | ANDERSON M G, SMITH R S, HAWES N L, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice[J]. Nat Genet, 2002, 30(1): 81–85. DOI: 10.1038/ng794 |
| [27] | LOFTUS S K, ANTONELLIS A, MATERA I, et al. Gpnmb is a melanoblast-expressed, MITF-dependent gene[J]. Pigment Cell Melanoma Res, 2009, 22(1): 99–110. DOI: 10.1111/pcr.2009.22.issue-1 |
| [28] | SCHALLREUTER K U, KOTHARI S, CHAVAN B, et al. Regulation of melanogenesis-controversies and new concepts[J]. Exp Dermatol, 2008, 17(5): 395–404. DOI: 10.1111/exd.2008.17.issue-5 |
| [29] | HAASS N K, SMALLEY K S M, LI L, et al. Adhesion, migration and communication in melanocytes and melanoma[J]. Pigment Cell Res, 2005, 18(3): 150–159. DOI: 10.1111/(ISSN)1600-0749 |
| [30] |
赵兵令, 王海东, 陈天直, 等. 小鼠毛囊不同生长周期中MITF下游色素相关基因的定位表达及相关性分析[J]. 中国生物化学与分子生物学报, 2017, 33(2): 198–206.
ZHAO B L, WANG H D, CHEN T Z, et al. Localization and correlation analysis of MITF downstream pigmentation-related genes at different growth cycle of mouse hair follicle[J]. Chinese Journal of Biochemistry and Molecular Biology, 2017, 33(2): 198–206. (in Chinese) |
| [31] | SCHEPSKY A, BRUSER K, GUNNARSSON G J, et al. The microphthalmia-associated transcription factor mitf interacts with β-catenin to determine target gene expression[J]. Mol Cell Biol, 2006, 26(23): 8914–8927. DOI: 10.1128/MCB.02299-05 |
| [32] | CHELI Y, LUCIANI F, KHALED M, et al. αMSH and cyclic AMP elevating agents control melanosome pH through a protein kinase A-independent mechanism[J]. J Biol Chem, 2009, 284(28): 18699–18706. DOI: 10.1074/jbc.M109.005819 |
| [33] | WALKER W P, GUNN T M. Shades of meaning:the pigment-type switching system as a tool for discovery[J]. Pigment Cell Melanoma Res, 2010, 23(4): 485–495. DOI: 10.1111/j.1755-148X.2010.00721.x |
| [34] | BUSCÀ R, ABBE P, MANTOUX F, et al. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes[J]. EMBO J, 2000, 19(12): 2900–2910. DOI: 10.1093/emboj/19.12.2900 |
| [35] | XU W D, GONG L M, HADDAD M M, et al. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9[J]. Exp Cell Res, 2000, 255(2): 135–143. DOI: 10.1006/excr.2000.4803 |
| [36] | NAKANO Y, SUZUKI Y, TAKAGI T, et al. Glycoprotein nonmetastatic melanoma protein B (GPNMB) as a novel neuroprotective factor in cerebral ischemia-reperfusion injury[J]. Neuroscience, 2014, 277: 123–131. DOI: 10.1016/j.neuroscience.2014.06.065 |
| [37] | BENTLEY N J, EISEN T, GODING C R. Melanocyte-specific expression of the human tyrosinase promoter:activation by the microphthalmia gene product and role of the initiator[J]. Mol Cell Biol, 1994, 14(12): 7996–8006. DOI: 10.1128/MCB.14.12.7996 |
| [38] |
赵兵令, 李亚楠, 陈天直, 等. GPNMB通过调控MITF下游色素相关基因表达影响黑色素细胞色素的生成[J]. 中国农业科学, 2017, 50(7): 1334–1342.
ZHAO B L, LI Y N, CHEN T Z, et al. GPNMB affects melanin synthesis in the melanocytes via MITF to regulate the downstream pigmental genes[J]. Scientia Agricultura Sinica, 2017, 50(7): 1334–1342. DOI: 10.3864/j.issn.0578-1752.2017.07.016 (in Chinese) |
| [39] | SATO S, ROBERTS K, GAMBINO G, et al. CBP/p300 as a co-factor for the Microphthalmia transcription factor[J]. Oncogene, 1997, 14(25): 3083–3092. DOI: 10.1038/sj.onc.1201298 |
| [40] | VACHTENHEIM J, BOROVANSKY J. "Transcription physiology" of pigment formation in melanocytes:central role of MITF[J]. Exp Dermatol, 2010, 19(7): 617–627. DOI: 10.1111/exd.2010.19.issue-7 |
| [41] | ANGHEL S I, CORREA-ROCHA R, BUDINSKA E, et al. Breast cancer suppressor candidate-1(BCSC-1) is a melanoma tumor suppressor that down regulates MITF[J]. Pigment Cell Melanoma Res, 2012, 25(4): 482–487. DOI: 10.1111/pcr.2012.25.issue-4 |
| [42] | PIERRAT M J, MARSAUD V, MAUVIEL A, et al. Expression of microphthalmia-associated transcription factor (MITF), which is critical for melanoma progression, is inhibited by both transcription factor GLI2 and transforming growth factor-β[J]. J Biol Chem, 2012, 287(22): 17996–18004. DOI: 10.1074/jbc.M112.358341 |
| [43] | LUCOTTE G, MERCIER G, DIéTERLEN F, et al. A decreasing gradient of 374F allele frequencies in the skin pigmentation gene SLC45A2, from the north of West Europe to North Africa[J]. Biochem Genet, 2010, 48(1-2): 26–33. DOI: 10.1007/s10528-009-9289-4 |
| [44] | DOOLEY C M, SCHWARZ H, MUELLER K P, et al. Slc45a2 and V-ATPase are regulators of melanosomal pH homeostasis in zebrafish, providing a mechanism for human pigment evolution and disease[J]. Pigment Cell Melanoma Res, 2013, 26(2): 205–217. DOI: 10.1111/pcmr.12053 |
| [45] | BIN B H, BHIN J, YANG S H, et al. Membrane-associated transporter protein (MATP) regulates melanosomal pH and influences tyrosinase activity[J]. PLoS One, 2015, 10(6): e0129273. DOI: 10.1371/journal.pone.0129273 |
| [46] | CUI R T, WIDLUND H R, FEIGE E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation[J]. Cell, 2007, 128(5): 853–864. DOI: 10.1016/j.cell.2006.12.045 |
| [47] | TADOKORO T, YAMAGUCHI Y, BATZER J, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation[J]. J Invest Dermatol, 2005, 124(6): 1326–1332. DOI: 10.1111/j.0022-202X.2005.23760.x |



