卵泡颗粒细胞的生长和凋亡,对卵泡的发育起着重要作用。卵泡发育是受颗粒细胞分泌的各种因子调节的复杂生物过程,尤其是类固醇激素如雌激素(E2) 和孕酮(P)[1-2]。类固醇激素的合成受一系列酶的控制,其中CYP19、CYP11和3β-HSD是其合成通路的关键酶[3-4]。抑制素是卵巢颗粒细胞中类固醇激素合成的重要调节因子[5-6],它可以通过调节类固醇激素合成相关基因(CYP11、3β-HSD和CYP19) 的表达或活性调控类固醇激素的合成,参与调控卵泡的生长发育与排卵,进而调节动物的繁殖性能[7-8]。
抑制素主要是卵泡颗粒细胞分泌的,它是由α、β两个不同的亚基组成,其中β亚基有A、B两种形式,α和βA亚基构成抑制素A(InhibinA),α和βB形成抑制素B(InhibinB)。抑制素的结合位点和受体位于垂体、卵泡膜细胞和卵泡颗粒细胞中[9-11],它可以通过内分泌和局部作用的方式调节卵泡发育[12-16]。许多研究表明,抑制素通过内分泌作用调节E2的分泌,从而调控垂体FSH的分泌[17-21]。另外,抑制素可通过局部调节作用增强LH刺激膜细胞雄激素的合成效应,促进雄激素的合成[22-23]。但是,颗粒细胞来源的抑制素对E2和P局部调节的影响结果还存在争议。研究发现,添加抑制素抗体促进牛和猪颗粒细胞分泌E2[24-26]。但是B.K.Campbell等[27]研究表明,抑制素的添加促进绵羊颗粒细胞产生E2,C.D.Smyth等[22]在大鼠卵泡中发现,免疫中和内源性的抑制素导致E2分泌的降低和P的增加,当抗体处理的卵泡中补充外源性抑制素时,E2分泌得到恢复,并且P减少。抑制素通过改变CYP11和CYP19的表达影响E2和P的分泌[25, 28]。另外,抑制素对灵长类动物颗粒细胞基础水平和促性腺激素诱导的E2的分泌没有影响[29]。抑制素对E2和P有重要的调节作用,但针对绵羊颗粒细胞的研究结果还比较少,且颗粒细胞来源的抑制素对E2和P局部作用的调控结果还不清楚。
本实验室已构建了靶向抑制素α亚基(Inhibin α-subunit, INHα)基因的siRNA,干扰效率达85%以上,并通过RNA干扰INHα的表达后对绵羊颗粒细胞的增殖和凋亡进行了大量研究,但关于RNA干扰INHα的表达及添加InhibinA后,对绵羊颗粒细胞E2和P的分泌及相关基因的变化还需要进一步研究。本研究以原代绵羊颗粒细胞为模型,通过siRNA干扰内源性INHα基因的表达和添加外源InhibinA两种处理,研究抑制素对绵羊颗粒细胞E2和P的分泌及相关基因(CYP11、3β-HSD和CYP19) 表达的影响,为研究卵泡抑制素对绵羊卵泡发育的局部调节作用奠定基础。
1 材料与方法 1.1 试验材料从唐县屠宰场采集1.0~1.5岁小尾寒羊的卵巢,立即放入含双抗的37 ℃生理盐水的保温瓶中,并在3 h内运回实验室。
1.2 方法 1.2.1 绵羊卵巢颗粒细胞的分离与培养根据J.Y.Peng等[30]的颗粒细胞分离方法,从绵羊卵泡分离并收集颗粒细胞,加入含10% FBS(Gibco)和1%青链霉素混合液的DMEM/F12(Gibco)培养液重悬细胞。调整细胞密度,于37 ℃、5% CO2条件下培养24 h后, 更换为含有1%的青链霉素混合液(Gibco)、0.2% BSA(Sigma)、1%的ITS(胰岛素-转铁因子-硒补充剂,Gibco)和0.1 μmol·L-1雄烯二酮(美伦)的DMEM/F-12培养液培养12 h后,添加InhibinA(200 ng·mL-1,ProSpec)对细胞进行刺激,并以未处理的细胞为空白对照(Control),每组设3个重复孔。
1.2.2 siRNA的设计合成和转染在GenBank上搜索绵羊INHα基因mRNA序列(NM_001308579.1),设计合成针对INHα基因的siRNA干扰序列(siINHα)和阴性对照(siNC),采用转染试剂LipofectamineTM RNAiMAX(Invitrogen),按说明书转染siINHα和siNC至绵羊颗粒细胞,每组设3个重复孔。处理48 h后, 提取总RNA进行检测。
1.2.3 雌激素和孕酮的测定RNA干扰组和InhibinA添加组分别处理细胞48 h后,收集培养液上清。采用P和E2 ELISA试剂盒(北京华英生物技术研究所)检测细胞培养液中E2(灵敏度 < 4 pg·mL-1,批内变异系数 < 15%,批间变异系数 < 15%)和P(灵敏度 < 0.1 ng·mL-1,批内变异系数 < 15%,批间变异系数 < 15%)的浓度,经显色后在酶标仪测定吸光值(OD值),通过拟合浓度—吸光度曲线,计算出待测细胞培养液中E2和P的含量。
1.2.4 qRT-PCR检测雌激素和孕酮分泌相关基因mRNA的表达干扰组和InhibinA添加组处理细胞48 h后,Trizol(Invitrogen)提取细胞总RNA,利用反转录试剂盒(TaKaRa),反转录为cDNA。使用荧光定量PCR仪(ABI Step One PlusTM)进行检测(SybrGreen qPCR mastermix试剂购自DBI)。以GAPDH基因为内参,采用2-△△CT的方法计算各检测基因的相对表达量。qRT-PCR检测目的基因INHα、E2和P分泌相关基因(CYP19、CYP11和3β-HSD)的相对表达量。引物序列见表 1。
|
|
表 1 引物序列 Table 1 Primer sequences |
采用SPSS 19.0的t检验对数据进行分析。试验数据以“平均值±标准差”表示。P<0.05表示差异显著。
2 结果 2.1 siRNA的干扰效率siRNA转染颗粒细胞48 h后,与阴性对照相比,颗粒细胞INHα mRNA的水平显著降低(P<0.05),抑制率为87%(表 2)。
|
|
表 2 转染后绵羊颗粒细胞INHα基因mRNA的相对表达水平 Table 2 The mRNA expression of INHα in sheep granulosa cells after transfection |
干扰组siRNA转染颗粒细胞48 h后,测定培养液中E2和P分泌量的结果见表 3。在转染48 h时,siRNA干扰组颗粒细胞E2和P的分泌量均显著低于阴性对照组(P < 0.05)。
|
|
表 3 干扰INHα对颗粒细胞中E2和P分泌的影响 Table 3 The effects of silencing INHα on the secretion of E2 (pg·mL-1) and P (ng·mL-1) in granulosa cells |
InhibinA处理颗粒细胞48 h后,E2和P的分泌量均显著高于空白对照组(P < 0.05)(表 4)。
|
|
表 4 InhibinA对颗粒细胞中E2和P分泌的影响 Table 4 The effects of inhibinA on the secretion of E2 and P in granulosa cells |
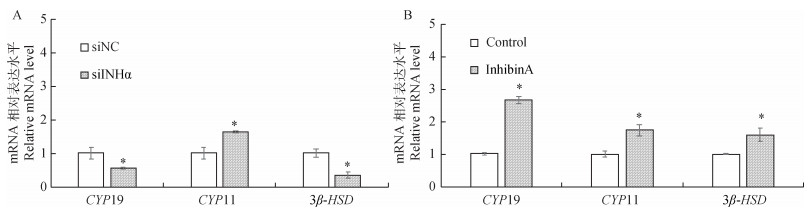
干扰组E2和P分泌相关基因的相对表达量结果见图 1A。siRNA转染后,干扰组中与E2分泌相关的芳香化酶CYP19基因相对表达量受到抑制,与阴性对照组相比差异显著(P < 0.05);与P分泌相关的3β-HSD基因相对表达量显著降低(P < 0.05),而CYP11的表达量显著升高(P < 0.05)。InhibinA添加组E2和P分泌相关基因的相对表达量结果见图 1B。与空白对照组相比,CYP19、CYP11和3β-HSD基因相对表达量均显著升高(P < 0.05)。
|
图 1 干扰INH α(A)和InhibinA(B)处理后绵羊颗粒细胞CYP19、CYP11和3β-HSD mRNA的表达 Figure 1 Effect of treatment with silencing INHα gene (A)and inhibinA (B) on the mRNA expressions of CYP19, CYP11 and 3β-HSD in sheep granulosa cells |
卵泡发育是受颗粒细胞分泌的各种因子调节的复杂生物过程,尤其受E2和P的调节。研究表明,抑制素可以调节E2和P的分泌[7, 31]。F.J.Chen等[32]研究发现,干扰鹅颗粒细胞INHα基因的表达后,E2的浓度降低。L.Y.Geng等[33]超表达INHα 48 h后发现,抑制素促进E2分泌,抑制P分泌;而96 h后,E2分泌受到抑制,P分泌量不变。可能是因为超表达的INHα片段能够作为抑制素的对抗物,模拟抑制素亚基对颗粒细胞的作用,对抗颗粒细胞自身分泌的抑制素对E2分泌的负反馈作用,促进颗粒细胞分泌E2。另外,抑制素抗体的添加,同样抑制FSH诱导的绵羊颗粒细胞和未成熟大鼠的整个卵泡中产生E2[22, 27],说明抑制素对E2有促进作用。本研究得出,干扰绵羊颗粒细胞中INHα基因的表达,抑制了E2和P的分泌,添加InhibinA处理绵羊颗粒细胞,促进了E2和P的分泌。但有研究发现,转基因小鼠过表达INHα,使得小鼠的排卵率降低,E2分泌减少[34-35]。在猪和牛颗粒细胞中添加抑制素抗体,阻断了猪和牛颗粒细胞产生的抑制素对E2的抑制效果,促进E2的分泌,说明抑制素对颗粒细胞产生的E2具有负反馈效应[24-26]。还有研究表明,抑制素对灵长类动物和大鼠颗粒细胞基础水平和FSH诱导的E2的分泌没有影响[29, 36]。以上研究结果不一致的原因,可能是由于物种、处理方法、培养基中添加的成分以及培养的时间不同造成的,也可能与颗粒细胞的分化阶段不同有关。
3.2 抑制素对颗粒细胞雌激素和孕酮分泌相关基因表达的影响E2和P在动物的生殖、发育、生长等方面起着重要作用,它的合成主要受CYP19、CYP11和3β-HSD的控制。研究表明,芳香化酶CYP19是雄激素向E2转化的关键酶,P的产生与3β-HSD和CYP11的表达有关[3]。L.P. Cai等[25]研究表明,添加INHα抗体,通过上调CYP19促进猪颗粒细胞E2的分泌。C.L.Lu等[28]研究发现InhibinA本身对大鼠颗粒细胞的CYP19和CYP11 mRNA基础水平没有影响,但抑制了FSH诱导的类固醇生成相关基因CYP19和CYP11 mRNA的表达,从而降低了E2和P的合成,这种抑制作用发生在cAMP通路的上游。本研究表明,干扰绵羊颗粒细胞中INHα基因的表达,显著下调CYP19和3β-HSD mRNA的表达,抑制E2和P的分泌;InhibinA处理颗粒细胞,显著上调CYP19、CYP11和3β-HSD的表达,促进E2和P的分泌,与以上研究结果一致。但干扰颗粒细胞中INHα基因的表达,CYP11 mRNA的表达上调与P分泌的变化不一致。M.Sahmi等[37]研究表明,3β-HSD对P的分泌起决定性作用,而CYP11的表达与P的分泌无关。研究表明,添加INHα抗体,免疫中和内源性抑制素的生物学活性,促进猪颗粒细胞分泌E2,但CYP19的表达上调不显著,认为E2的合成和分泌可能还涉及CYP19以外的因子[24]。抑制素对E2和P的调控作用涉及多因子参与,其调控机制比较复杂,造成以上基因表达差异的原因还需进一步的研究。
4 结论RNA干扰INHα基因的表达后,抑制了绵羊颗粒细胞E2和P的分泌,显著下调CYP19和3β-HSD mRNA的表达,上调CYP11 mRNA的表达;InhibinA的添加,促进了绵羊颗粒细胞E2和P的分泌,显著上调CYP19、CYP11和3β-HSD mRNA的表达。抑制素通过改变类固醇生成相关基因的表达,进而调控绵羊颗粒细胞类固醇激素的分泌。
| [1] | KNIGHT P G, SATCHELL L, GLISTER C. Intra-ovarian roles of activins and inhibins[J]. Mol Cell Endocrinol, 2012, 359(1-2): 53–65. DOI: 10.1016/j.mce.2011.04.024 |
| [2] | RIAZ H, DONG P, SHAHZAD M, et al. Constitutive and follicle-stimulating hormone-induced action of somatostatin receptor-2 on regulation of apoptosis and steroidogenesis in bovine granulosa cells[J]. J Steroid Biochem Mol Biol, 2014, 141: 150–159. DOI: 10.1016/j.jsbmb.2014.02.001 |
| [3] | LAVOIE H A, KING S R. Transcriptional regulation of steroidogenic genes:STARD1, CYP11A1 and HSD3B[J]. Exp Biol Med (Maywood), 2009, 234(8): 880–907. DOI: 10.3181/0903-MR-97 |
| [4] | STOCCO D M, CLARK B J. Regulation of the acute production of steroids in steroidogenic cells[J]. Endocr Rev, 1996, 17(3): 221–244. |
| [5] | KNIGHT P G, GLISTER C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary[J]. Reproduction, 2001, 121(4): 503–512. DOI: 10.1530/rep.0.1210503 |
| [6] | HILLIER S G. Regulatory functions for inhibin and activin in human ovaries[J]. J Endocrinol, 1991, 131(2): 171–175. DOI: 10.1677/joe.0.1310171 |
| [7] | HILLIER S G, TSONIS C G, WICKINGS E J, et al. Inhibition of FSH-stimulated granulosa cell function by a synthetic fragment of the porcine inhibin alpha-subunit:evidence for involvement of GnRH receptors[J]. J Endocrinol, 1987, 113(2): R3–R5. DOI: 10.1677/joe.0.113R003 |
| [8] | SCHNEYER A L, SLUSS P M, WHITCOMB R W, et al. Precursors of α-inhibin modulate follicle-stimulating hormone receptor binding and biological activity[J]. Endocrinology, 1991, 129(4): 1987–1999. DOI: 10.1210/endo-129-4-1987 |
| [9] | LEWIS K A, GRAY P C, BLOUNT A L, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling[J]. Nature, 2000, 404(6776): 411–414. DOI: 10.1038/35006129 |
| [10] | HERTAN R, FARNWORTH P G, FITZSIMMONS K L, et al. Identification of high affinity binding sites for inhibin on ovine pituitary cells in culture[J]. Endocrinology, 1999, 140(1): 6–12. DOI: 10.1210/endo.140.1.6440 |
| [11] | MACCONELL L A, LEAL A M O, VALE W W. The distribution of betaglycan protein and mRNA in rat brain, pituitary, and gonads:implications for a role for betaglycan in inhibin-mediated reproductive functions[J]. Endocrinology, 2002, 143(3): 1066–1075. DOI: 10.1210/endo.143.3.8707 |
| [12] | HILLIER S G, MIRÓ F. Inhibin, activin, and follistatin. Potential roles in ovarian physiology[J]. Ann N Y Acad Sci, 1993, 687: 29–38. DOI: 10.1111/nyas.1993.687.issue-1 |
| [13] | VALE W, HSUEH A, RIVIER C, et al. The Inhibin/Activin family of hormones and growth factors[M]//SPORN M B, ROBERTS A B. Peptide Growth Factors and Their Receptors Ⅱ. Berlin Heidelberg:Springer, 1990:211-248. |
| [14] | FINDLAY J K, DRUMMOND A E, DYSON M, et al. Production and actions of inhibin and activin during folliculogenesis in the rat[J]. Mol Cell Endocrinol, 2001, 180(1-2): 139–144. DOI: 10.1016/S0303-7207(01)00521-4 |
| [15] | BILEZIKJIAN L M, BLOUNT A L, LEAL A M O, et al. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin[J]. Mol Cell Endocrinol, 2004, 225(1-2): 29–36. DOI: 10.1016/j.mce.2004.02.010 |
| [16] | KADARIYA I, WANG J X, REHMAN Z U, et al. RNAi-mediated knockdown of inhibin α subunit increased apoptosis in granulosa cells and decreased fertility in mice[J]. J Steroid Biochem Mol Biol, 2015, 152: 161–170. DOI: 10.1016/j.jsbmb.2015.05.006 |
| [17] | DAN X G, LIU X R, HAN Y G, et al. Effect of the novel DNA vaccine fusing inhibin α (1-32) and the RF-amide related peptide-3 genes on immune response, hormone levels and fertility in Tan sheep[J]. Anim Reprod Sci, 2016, 164: 105–110. DOI: 10.1016/j.anireprosci.2015.11.018 |
| [18] | RAMASWAMY S, POHL C R, MCNEILLY A S, et al. The time course of follicle-stimulating hormone suppression by recombinant human inhibin A in the adult male rhesus monkey (Macaca mulatta)[J]. Endocrinology, 1998, 139(8): 3409–3415. DOI: 10.1210/endo.139.8.6125 |
| [19] | LIU Y P, MAO X B, WEI Y M, et al. Studies on enhancing embryo quantity and quality by immunization against inhibin in repeatedly superovulated Holstein heifers and the associated endocrine mechanisms[J]. Anim Reprod Sci, 2013, 142(1-2): 10–18. DOI: 10.1016/j.anireprosci.2013.08.005 |
| [20] | BAHARELDIN-ALI A, QIN G S, GUO R H, et al. Endocrine and ovarian responses in water buffalo cows immunized against inhibin and subjected to the Ovsynch protocol[J]. J Integr Agric, 2015, 14(9): 1827–1837. DOI: 10.1016/S2095-3119(15)61034-6 |
| [21] | LIU Q, REHMAN Z U, LIU J J, et al. Nasal immunization with inhibin DNA vaccine delivered by attenuated Salmonella choleraesuis for improving ovarian responses and fertility in cross-bred buffaloes[J]. Rerod Domest Anim, 2017, 52(2): 189–194. DOI: 10.1111/rda.2017.52.issue-2 |
| [22] | SMYTH C D, GOSDEN R G, MCNEILLY A S, et al. Effect of inhibin immunoneutralization on steroidogenesis in rat ovarian follicles in vitro[J]. J Endocrinol, 1994, 140(3): 437–443. DOI: 10.1677/joe.0.1400437 |
| [23] | YOUNG J M, MCNEILLY A S. Inhibin removes the inhibitory effects of activin on steroid enzyme expression and androgen production by normal ovarian thecal cells[J]. J Mol Endocrinol, 2012, 48(1): 49–60. DOI: 10.1530/JME-11-0134 |
| [24] | LEI M M, CAI L P, LI H, et al. Transcriptome sequencing analysis of porcine granulosa cells treated with an anti-inhibin antibody[J]. Reprod Biol, 2017, 17(1): 79–88. DOI: 10.1016/j.repbio.2017.01.002 |
| [25] | CAI L P, SUN A D, LI H, et al. Molecular mechanisms of enhancing porcine granulosa cell proliferation and function by treatment in vitro with anti-inhibin alpha subunit antibody[J]. Reprod Biol Endocrinol, 2015, 13: 1–10. DOI: 10.1186/1477-7827-13-1 |
| [26] | JIMENEZ-KRASSEL F, WINN M E, BURNS D, et al. Evidence for a negative intrafollicular role for inhibin in regulation of estradiol production by granulosa cells[J]. Endocrinology, 2003, 144(5): 1876–1886. DOI: 10.1210/en.2002-221077 |
| [27] | CAMPBELL B K, BAIRD D T. Inhibin A is a follicle stimulating hormone-responsive marker of granulosa cell differentiation, which has both autocrine and paracrine actions in sheep[J]. J Endocrinol, 2001, 169(2): 333–345. DOI: 10.1677/joe.0.1690333 |
| [28] | LU C L, YANG W, CHEN M, et al. Inhibin A inhibits follicle-stimulating hormone (FSH) action by suppressing its receptor expression in cultured rat granulosa cells[J]. Mol Cell Endocrinol, 2009, 298(1-2): 48–56. DOI: 10.1016/j.mce.2008.09.039 |
| [29] | HILLIER S G, MIRÓ F. Local regulation of primate granulosa cell aromatase activity[J]. J Steroid Biochem Mol Biol, 1993, 44(4-6): 435–439. DOI: 10.1016/0960-0760(93)90247-T |
| [30] | PENG J Y, XIN H Y, HAN P, et al. Expression and regulative function of tissue inhibitor of metalloproteinase 3 in the goat ovary and its role in cultured granulosa cells[J]. Mol Cell Endocrinol, 2015, 412: 104–115. DOI: 10.1016/j.mce.2015.06.001 |
| [31] | MIRÓ F, HILLIER S G. Relative effects of activin and inhibin on steroid hormone synthesis in primate granulosa cells[J]. J Clin Endocrinol Metab, 1992, 75(6): 1556–1561. |
| [32] | CHEN F J, JIANG X P, CHEN X P, et al. Effects of downregulation of inhibin α gene expression on apoptosis and proliferation of goose granulosa cells[J]. J Genet Genomics, 2007, 34(12): 1106–1113. DOI: 10.1016/S1673-8527(07)60126-X |
| [33] | GENG L Y, FANG M, YI J M, et al. Effect of overexpression of inhibin α (1-32) fragment on bovine granulosa cell proliferation, apoptosis, steroidogenesis, and development of co-cultured oocytes[J]. Theriogenology, 2008, 70(1): 35–43. DOI: 10.1016/j.theriogenology.2008.02.013 |
| [34] | CHO B N, MCMULLEN M L, PEI L, et al. Reproductive deficiencies in transgenic mice expressing the rat inhibin α-subunit gene[J]. Endocrinology, 2001, 142(11): 4994–5004. DOI: 10.1210/endo.142.11.8481 |
| [35] | MCMULLEN M L, CHO B N, YATES C J, et al. Gonadal pathologies in transgenic mice expressing the rat inhibin α-subunit[J]. Endocrinology, 2001, 142(11): 5005–5014. DOI: 10.1210/endo.142.11.8472 |
| [36] | HUTCHINSON L A, FINDLAY J K, DE VOS F L, et al. Effects of bovine inhibin, transforming growth factor-β and bovine Activin-A on granulosa cell differentiation[J]. Biochem Biophys Res Commun, 1987, 146(3): 1405–1412. DOI: 10.1016/0006-291X(87)90806-0 |
| [37] | SAHMI M, NICOLA E S, SILVA J M, et al. Expression of 17β-and 3β-hydroxysteroid dehydrogenases and steroidogenic acute regulatory protein in non-luteinizing bovine granulosa cells in vitro[J]. Mol Cell Endocrinol, 2004, 223(1-2): 43–54. DOI: 10.1016/j.mce.2004.05.010 |



