副流感病毒3型(PIV3) 属于副黏病毒科呼吸道病毒属成员,为有囊膜的单股负链RNA病毒。该属成员还包括人副流感病毒1型和3型(HPIV1、HPIV3)、仙台病毒(SV)和牛副流感病毒3型(BPIV3)[1]。HPIV1和HPIV3是导致婴幼儿上下呼吸道感染的重要病原[2];SV常造成实验室幼鼠的大量死亡;BPIV3可引起牛的“运输热”,当有致病性细菌或支原体继发感染时,会引起犊牛大批死亡,给养牛业带来重大的经济损失[3]。
然而,前期的研究中关于羊群感染PIV3鲜有报道。本研究室自2013年起在江苏、安徽等地的山羊养殖场中检测到一种新型PIV3,发病羊群临诊表现以呼吸道症状为主,将采集的病料经RT-PCR检测和测序分析,证实该病毒与HPIV3和BPIV3均存在较大差异,在进化上处于独立的分支,命名为山羊副流感病毒3型(CPIV3)[4-5]。致病性研究发现,攻毒后的山羊出现喷嚏、咳嗽、流鼻涕、眼分泌物增多及呼吸困难等临床症状;鼻拭子和肛拭子检测到持续排毒;剖检观察到肺的组织病理损伤,证实了CPIV3分离株对山羊具有较强的致病性[4, 6]。
微小核糖核酸(microRNA, miRNA)是一类长度19~24 nt的内源性非编码RNA,miRNA广泛存在于真核生物中,并通过与靶基因端非翻译序列互补结合促进靶基因降解或抑制其翻译,从而发挥基因转录后的调节功能[7-8]。研究表明,miRNA分子广泛参与调控细胞分化、增殖、代谢及凋亡的各个环节,并与动物机体生殖发育、肿瘤形成及病毒感染过程密切相关[9]。病毒感染能使宿主细胞miRNA表达谱发生改变,从而影响病毒的增殖及致病力[10]。猪伪狂犬病毒(PRV)感染猪树突状细胞(DC)可改变miR-27b、miR-29a和miR-30e-3p的表达,通过分析发现这些差异表达的miRNA靶基因可能与病毒潜伏感染有重大关系[11];N. J. Thornburg等研究发现,呼吸道合胞病毒(RSV)感染支气管上皮细胞,导致let-7i和miR-30b表达上调,通过操控NF-κB信号通路抑制病毒感染[12]。因此,本研究使用CPIV3感染MDBK细胞,通过高通量测序技术筛选出感染细胞和正常细胞中差异表达的miRNA,并对部分差异表达的miRNA进一步验证分析,以期为揭示CPIV3的致病机制及抗病毒研究提供重要的依据。
1 材料与方法 1.1 病毒与主要试剂CPIV3 JS2013 MDBK细胞适应株为本实验室分离并保存[4];MDBK细胞购自中国兽医药品监察所;miRcute miRNA提取分离试剂盒、miRcute miRNA cDNA第一链合成试剂盒和miRcute miRNA荧光定量检测试剂盒(SYBR Green)均购自TIANGEN公司。
1.2 测序样本的制备将MDBK细胞铺于60 mm细胞培养皿中,待其长满单层后接种10 MOI的CPIV3 JS2013,37 ℃孵育1 h后弃掉病毒液,添加含2% FBS的DMEM继续培养,24 h后收获细胞冻存于-70 ℃备用,同步设正常MDBK细胞为对照。感染试验重复3次。
1.3 HiSeq深度测序将收获的细胞样品送往华大基因公司进行高通量测序,其文库构建及测序由华大基因公司使用Illumina Genome Analyzer (Solexa平台)完成。
1.4 MDBK细胞miRNA的提取将收获的MDBK细胞分别加入裂解液1 mL,然后按照试剂盒说明书方法提取细胞中的miRNA,用分光光度计测定miRNA提取物的OD260 nm/ OD280 nm和OD260 nm /OD 230 nm值,得出浓度和纯度,并用甲醛变性琼脂糖凝胶电泳检测RNA的完整性。
1.5 荧光定量RT-qPCR验证差异表达的miRNA根据高通量测序结果,选择5个检出量高且差异表达显著的miRNA:bta-miR-129、bta-miR-1246、bta-miR-2478、bta-miR-2904和bta-miR-3613a,分别合成正向引物和内参引物5S rRNA(表 1),通用下游引物为荧光定量试剂盒自带,miRNA逆转录及qPCR均按照试剂盒说明书进行。采用2-ΔΔCt法计算MDBK细胞中miRNA的相对表达量。
|
|
表 1 扩增miRNA的相对定量PCR特异性引物 Table 1 Primers used to detect miRNA expression using RT-qPCR |
利用Bioanalyzer检测接毒细胞和正常细胞总RNA完整性,结果显示RNA Intergrity Number (RIN)=10和10,且28S/18S=2.2和2.3,表明样品完整性好,质量合格。CPIV3-infected样品和Mock-infected样品的电泳峰和对应的凝胶图见图 1,约25 nt处的峰为marker peak,对应凝胶图下方的绿带;近200 nt处的峰为sRNA peak,包括miRNA、tRNA、5S rRNA和5.8S rRNA等;约2 000 nt处的峰为18S rRNA peak;约4 000 nt处的峰为28S rRNA peak。
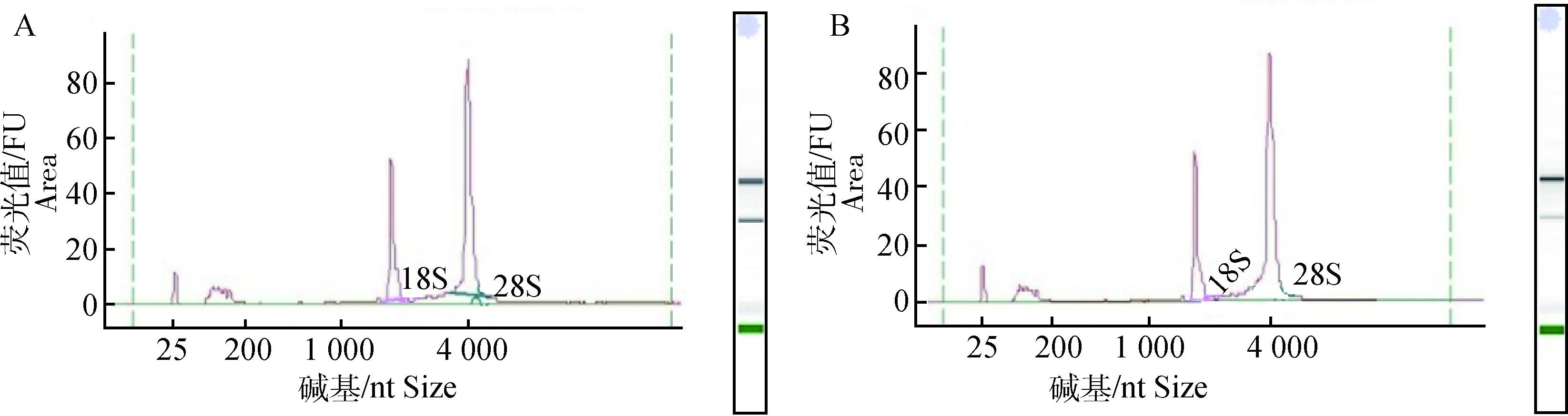
|
A.感染样品分析;B.未感染样品分析 A. The analysis of CPIV3-infected sample; B. The analysis of Mock-infected sample 图 1 细胞总RNA的完整性检测 Figure 1 The integrality of cellular total RNA |
两个测序样品sRNA(18~30 nt)总质量分别为12 853 008和13 509 329,剔除低质量的reads获得的clean read分别占原样本的95.26%和96.50%(表 2)。
|
|
表 2 高通量测序数据质量统计 Table 2 Data cleaning of the sequencing data |
CPIV3-infected和Mock-infected两个样品高质量sRNA长度分布在21~24 nt,分别占总样品的82.15%和90.83%。22和23 nt长度的sRNA丰度最高,这与miRNA大小是一致的,说明样品中含有大量的miRNA,见图 2。
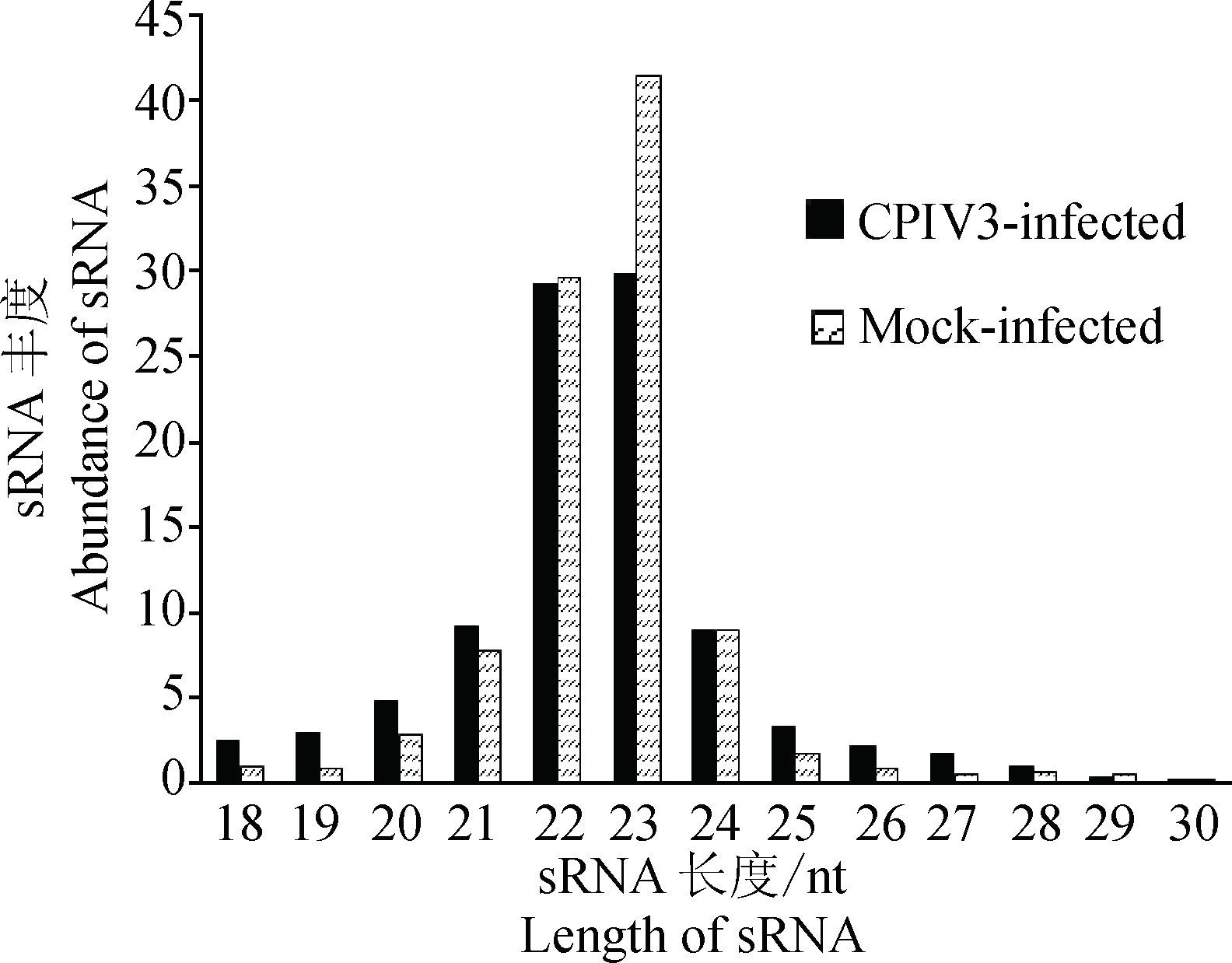
|
图 2 CPIV3感染和未感染样本的sRNA长度分布 Figure 2 Length distributions of the clean reads of the sequence |
两个测序样品sRNA(18~30 nt)总质量分别为12 853 008和13 509 329,剔除低质量的reads获得的clean reads分别为12 221 472和12 893 853。经GenBank和Rfam(10.1) 数据库来注释测序获得的序列,可将clean reads分为miRNA、rRNA、scRNA、snRNA、snoRNA、srpRNA、tRNA、Exon antisense、Exon sense、Intron antisense、Intron sense、repeat。将得到的sRNA与miRBase中该物种已知的miRNA前体/miRNA成熟体序列进行比对,其中感染样品和未感染样品中的miRNA数量分别为5 346和5 311个(表 3)。
|
|
表 3 sRNA在感染样品中和未感染样品中的注释 Table 3 Summary of deep sequencing data for sRNA in CPIV3-infected and Mock-infected sample |
将获得的miRNA与miRBase数据库比对,得出已知miRNA数量分别为348和360个,将两个样本已知miRNA进行差异表达聚类分析,图 3可见CPIV3感染MDBK细胞后出现了大量已知miRNA差异表达,范围在2-4~24之间。从图 4可以看出,有18个miRNA(红点)是显著上调,有19个miRNA(绿点)显著下调。从表 4可看出,大多数差异显著的miRNA碱基数集中在19~25 nt,且5′端起始碱基以A或U居多。
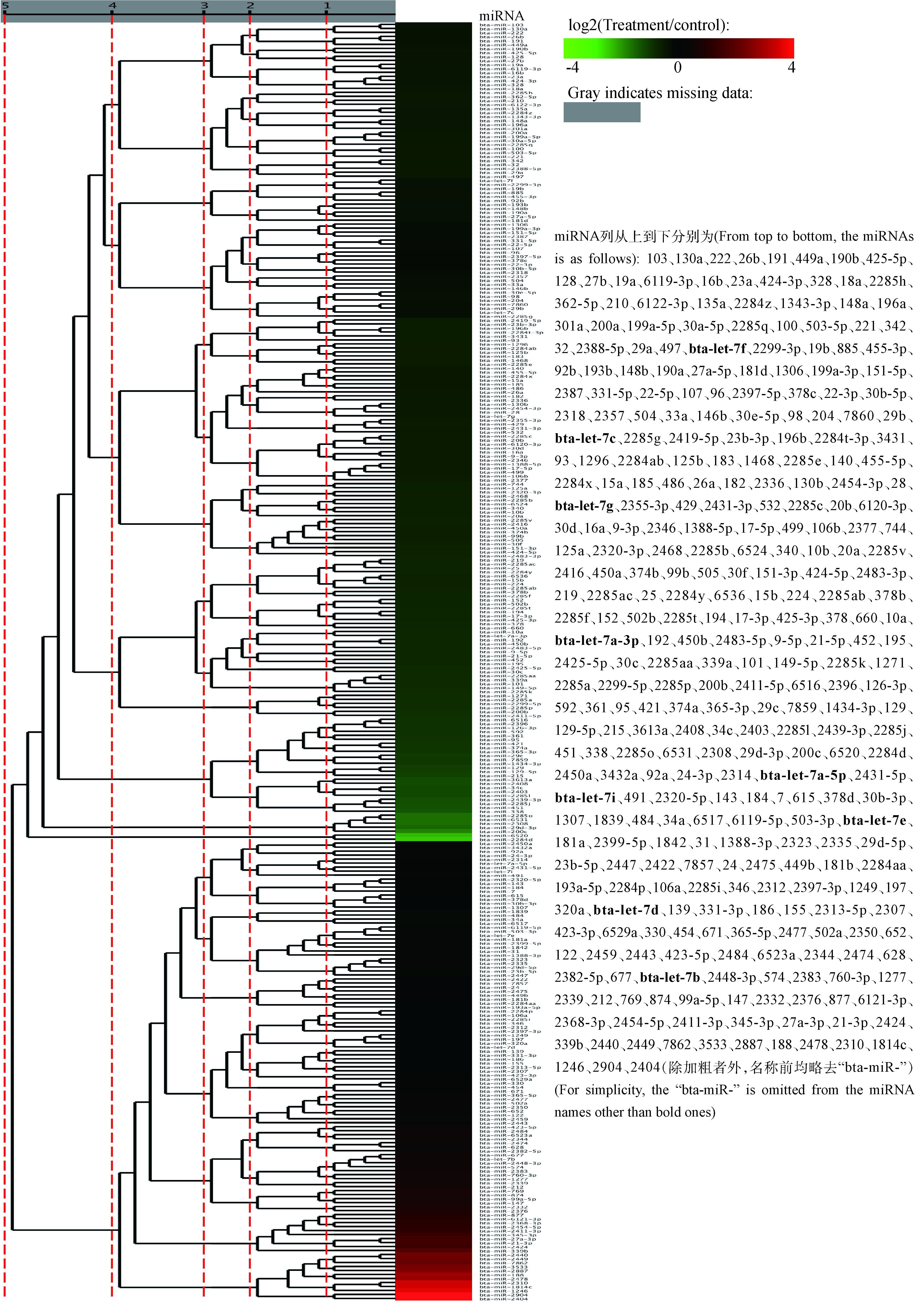
|
图 3 差异表达的已知miRNA聚类图 Figure 3 Cluster analysis of differential expression known miRNA between the CPIV3-infected and Mock-infected samples |
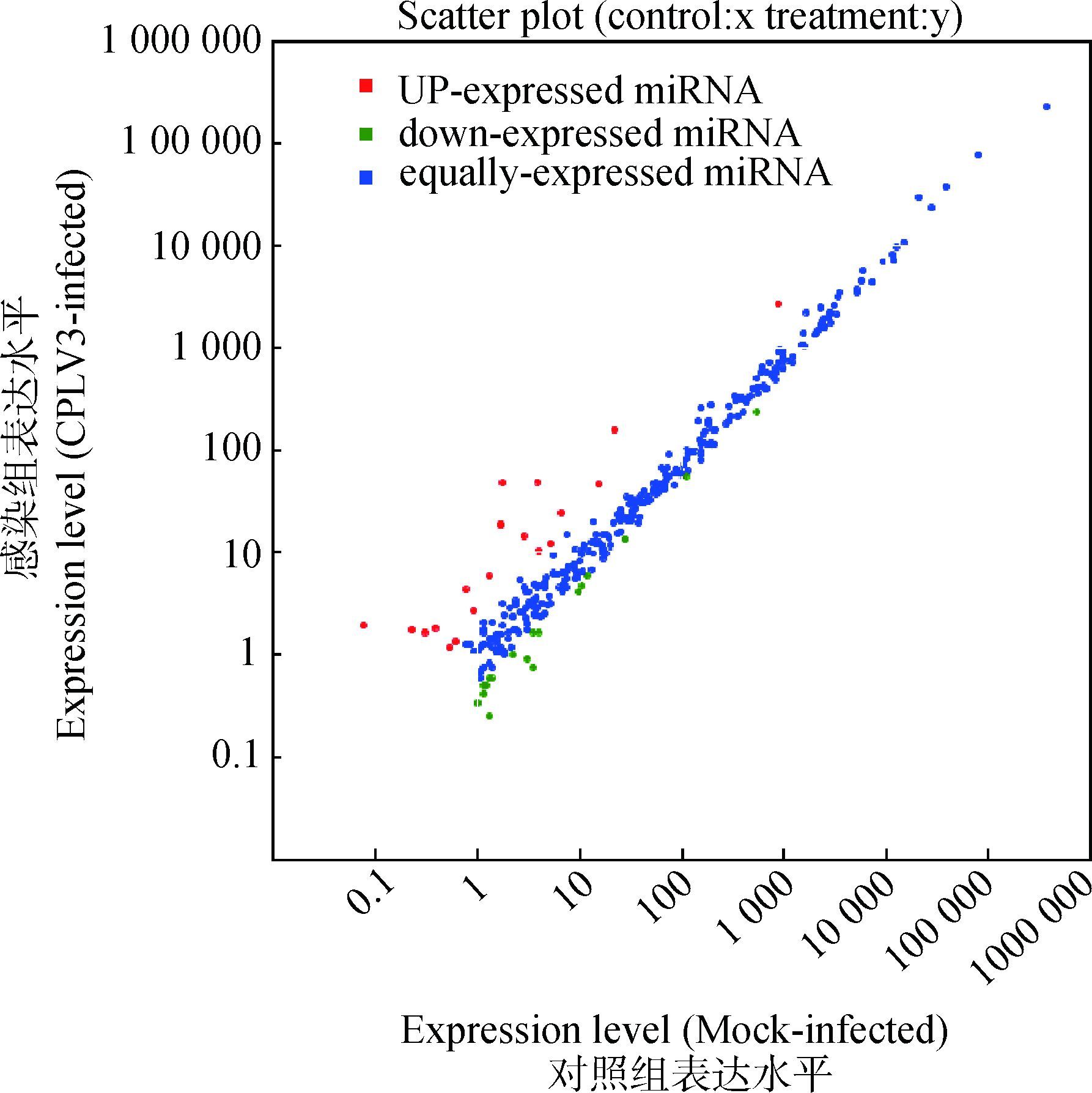
|
红色表示感染与未感染CPIV3细胞的miRNA比值大于2;蓝色表示比值介于1/2~2;绿色表示比值小于1/2 Red, miRNAs with ratio > 2 (CPIV3-infected/Mock-infected in expression); blue, miRNAs with 1/2≤ ratio ≤2, green, miRNAs with ratio < 1/2 图 4 样品间已知miRNA差异表达分析 Figure 4 Differential expression of host known miRNA between the CPIV3-infected and Mock-infected samples |
|
|
表 4 差异表达的miRNA情况 Table 4 Detail of differentially expressed miRNA in CPIV3-infected and Mock-infected sample |
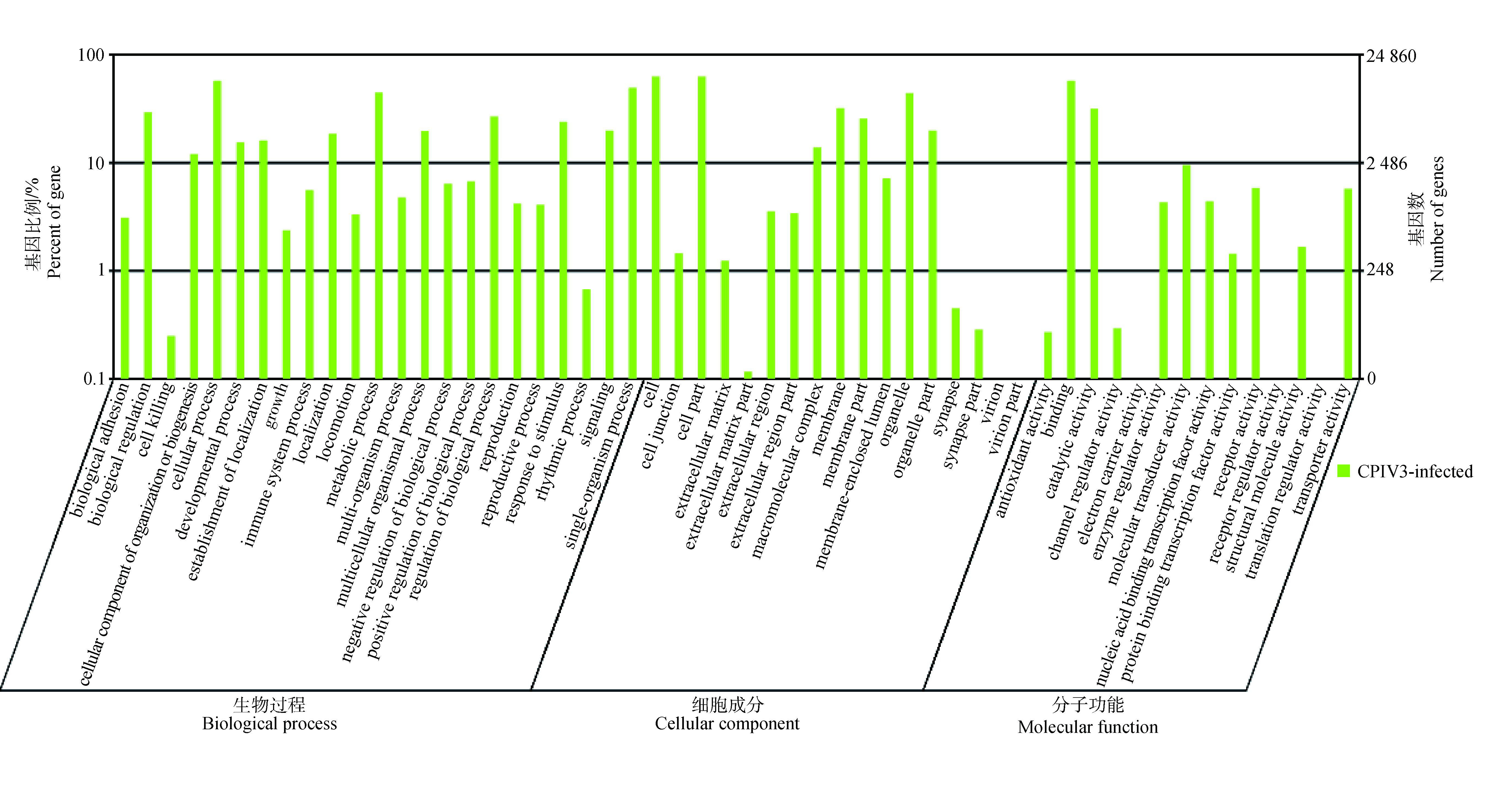
通过GO功能注释对miRNA预测的靶基因的分布和种类来推断miRNA的功能。从图 5可以看出,miRNA预测的靶基因参与细胞新陈代谢、细胞生物调控及免疫调节等生物过程。KEGG信号通路分析发现,这些靶基因参与多种细胞的信号通路,包括代谢通路、黏合斑、细胞黏附分子、B细胞受体信号通路、MAPK信号通路、钙离子信号通路和趋化因子信号通路等。
|
图 5 miRNA靶基因的GO的注释 Figure 5 Gene ontology statistics of miRNA target gene |
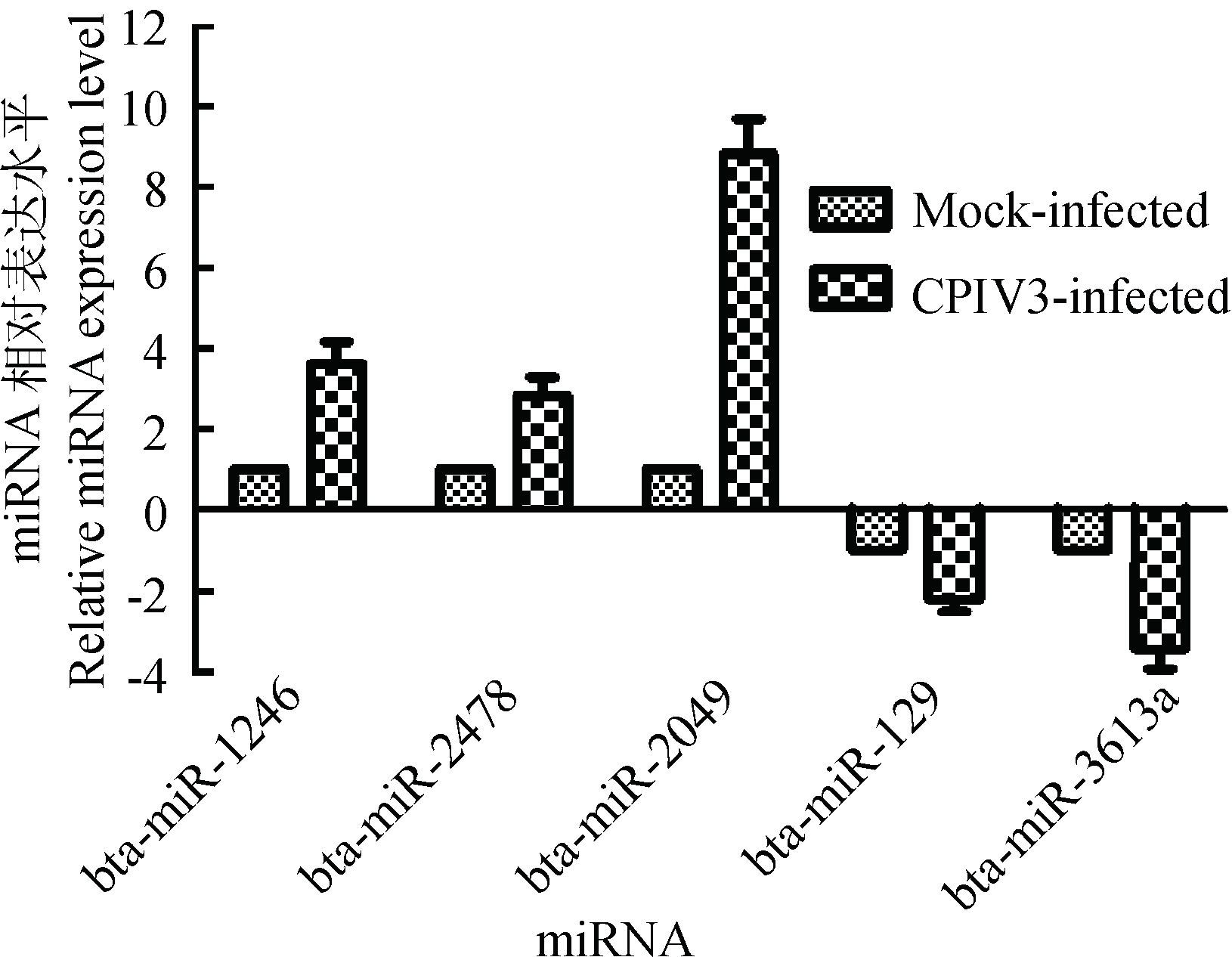
选择5个差异极显著的上调和下调的miRNA,采用5S rRNA作为内参,通过特异性引物对差异表达的miRNA进行RT-qPCR验证。试验进行3次独立重复,检测结果与高通量测序结果一致,见图 6。
|
图 6 差异miRNA的RT-qPCR验证 Figure 6 Validation of differentially expressed host miRNAs using RT-qPCR |
近年来,高通量测序已经成为分析动物、植物和病毒miRNA表达谱的有效方法。基于高通量测序技术的sRNA数字化分析模式具有通量高、对样品需求量少、精确度高及操作简单等优点,能够快速而全面的进行各样品之间的miRNA差异表达分析。细胞内源性miRNA是调节病毒宿主细胞的关键因子,然而miRNA的表达受到病毒感染而失调,一些细胞miRNA能通过调节宿主的免疫反应和病毒靶位而抑制病毒增殖[13];有些miRNA能够通过改变细胞内环境启动病毒的复制[14]。CPIV3是危害养羊业的重要呼吸道病原之一,在应激等条件下能诱导严重的疾病[15]。MDBK细胞是本实验室用于分离CPIV3病原的细胞系,在进行miRNA表达谱研究时使用10 MOI的病毒液感染宿主细胞,24 h后收获细胞,这可使每个细胞接受病毒刺激,参与调控miRNA的表达变化,同时可避免RNA降解和保障sRNA文库的质量[16-17],因此本研究选择该接种剂量和时间点进行miRNA表达谱分析。将CPIV3感染MDBK细胞24 h后通过高通量测序发现,感染细胞样品和正常细胞样品分别获得12 221 472和12 893 853个clean reads,这些序列中有37个表达差异显著的已知miRNA和115个表达差异显著的新miRNA。因此,miRNA在CPIV3感染MDBK细胞中扮演着重要的作用。
外界病原微生物入侵感染可诱导宿主细胞产生一系列的sRNA群,包括siRNAs、miRNA和piRNAs等[10, 18]。在本研究中,两个测序样品大多数clean reads的长度均在21~24 nt,在感染样品中共鉴定出348个已知miRNA和405个新miRNA;正常细胞样品中已知miRNA和新miRNA数量分别为360个和444个。同时,鉴定的miRNA碱基数集中在19~25 nt,首位碱基的偏爱性以A或U居多,这与J.J.Sun等研究牛源宿主细胞miRNA表达谱的结果一致,增加了鉴定结果的准确性[19]。
病毒感染改变宿主细胞的miRNA表达谱,这些变化可能是机体用来抵抗病毒复制的宿主细胞先天性免疫应答;亦或病毒通过改变某个通路的miRNA,而利于病毒增殖[20]。K. D. Taganov等研究表明,miR-146a的靶基因可调节肿瘤坏死因子受体因子6(TRAF6) 和白细胞介素1受体相关激酶(IRAK1),而它们在调控脂多糖(LPS)介导先天性免疫应答中发挥重要作用[21]。A. S. Collins等发现,miR-19a可调节SOCS 3的表达,而SOCS 3是细胞因子信号转导的重要抑制剂[22]。同时,miRNA在调节病毒感染应答中发挥重要作用[23]。比如,EB病毒感染人B细胞时,miR-155可上调1 000倍以上,高表达的miR-155可引发B细胞白血病和淋巴瘤[24];猪流感病毒H1N1亚型亦可影响miRNA的表达谱,该病毒感染A549细胞时有39个miRNA上调和13个miRNA下调,靶基因分析表明,差异表达的miRNA参与调节免疫反应和细胞增殖[25]。Q. Fu等将牛病毒性腹泻病毒(BVDV)接种MDBK细胞,感染细胞的miR-29b上调2.3倍以上,进一步研究发现miR-29b是通过下调自噬相关蛋白ATG14和ATG9A的表达来抑制BVDV增殖[26];随后该研究团队又发现了miR-29b能与Caspase-7及核凋亡诱导因子蛋白1(NAIF1) 靶向作用从而减小细胞凋亡作用,并且降低BVDV在MDBK细胞中的复制能力[27]。在本研究中,miR-29c和miR-29d-3p在感染细胞中均出现不同程度的下调,说明miR-29在CPIV3感染MDBK细胞中发挥了一定作用。W. W. Deng等和Y. R. Wu等均发现过表达miR-21能抑制细胞凋亡[28-29],J. Huang等研究得出miR-21能抑制PRV增殖[30],本试验发现,miR-21-3p在CPIV3感染MDBK细胞中表达量提高约3倍,同时J. L. Duan等和G. H. Zhang等的研究得出,过表达miR-129可促进细胞凋亡[31-32],而在CPIV3感染MDBK细胞样品中miR-129表达量降低约2倍,因此miR-21和miR-129是否参与CPIV3诱导的细胞凋亡及调控CPIV3在细胞中的增殖有待进一步证实。随后笔者选择深度测序检出量高且表达差异显著的5个miRNA进行RT-qPCR验证,试验结果与高通量分析有很好的一致性。使用靶基因在线预测软件分析发现(http://www.targetscan.org/),这些miRNA参与细胞凋亡、免疫调节和抗病毒反应等多种信号通路,其功能有待进一步试验论证。
通过GO富集和信号通路分析发现,CPIV3感染细胞中差异表达的37个已知miRNA和108个新miRNA分别预测到17 446个和52 598靶基因,这些靶基因参与细胞新陈代谢、细胞生物调控及免疫调节等生物过程。KEGG信号通路分析发现,这些靶基因参与多种细胞的信号通路,包括代谢通路、黏合斑、细胞黏附分子、B细胞受体信号通路、MAPK信号通路、钙离子信号通路和趋化因子信号通路等,其中MAPK信号通路和钙离子信号通路与细胞增殖和凋亡密切相关[33-34],因此也可能会影响CPIV3在MDBK细胞中的复制。本试验不仅为阐明内源性miRNA对CPIV3感染的分子调控提供了科学数据,而且为抗CPIV3的分子靶标的研发奠定了基础。
4 结论通过高通量测序分析探讨了CPIV3接种MDBK细胞的miRNA表达谱变化。鉴定了显著变化的37个已知miRNA和108个新miRNA,靶基因预测与功能分析发现这些miRNA调控CPIV3的复制以及宿主细胞的先天免疫应答。
| [1] | SHI H F, ZHU Y M, DONG X M, et al. Pathogenesis of a genotype C strain of bovine parainfluenza virus type 3 infection in albino guinea pigs[J]. Virus Res, 2014, 188: 1–7. DOI: 10.1016/j.virusres.2014.03.017 |
| [2] | KARRON R A, THUMAR B, SCHAPPELL E, et al. Evaluation of two chimeric bovine-human parainfluenza virus type 3 vaccines in infants and young children[J]. Vaccine, 2012, 30(26): 3975–3981. DOI: 10.1016/j.vaccine.2011.12.022 |
| [3] | HORWOOD P F, GRAVEL J L, MAHONY T J. Identification of two distinct bovine parainfluenza virus type 3 genotypes[J]. J Gen Virol, 2008, 89(Pt 7): 1643–1648. |
| [4] | LI W L, MAO L, CHENG S P, et al. A novel parainfluenza virus type 3 (PIV3) identified from goat herds with respiratory diseases in eastern China[J]. Vet Microbiol, 2014, 174(1-2): 100–106. DOI: 10.1016/j.vetmic.2014.08.027 |
| [5] | YANG L L, LI W L, MAO L, et al. Analysis on the complete genome of a novel caprine parainfluenza virus 3[J]. Infect Genet Evol, 2016, 38: 29–34. DOI: 10.1016/j.meegid.2015.11.027 |
| [6] | LI W L, HAO F, Mao L, et al. Pathogenicity and horizontal transmission studies of caprine parainfluenza virus type 3 JS2013 strain in goats[J]. Virus Res, 2016, 223: 80–87. DOI: 10.1016/j.virusres.2016.06.021 |
| [7] | BARTEL D P. MicroRNAs: genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281–297. DOI: 10.1016/S0092-8674(04)00045-5 |
| [8] | BARTEL D P. MicroRNAs: target recognition and regulatory functions[J]. Cell, 2009, 136(2): 215–233. DOI: 10.1016/j.cell.2009.01.002 |
| [9] | KLOOSTERMAN W P, PLASTERK R H A. The diverse functions of microRNAs in animal development and disease[J]. Dev Cell, 2006, 11(4): 441–450. DOI: 10.1016/j.devcel.2006.09.009 |
| [10] | XING S S, DU J Z, GAO S D, et al. Analysis of the miRNA expression profile in an Aedes albopictus cell line in response to bluetongue virus infection[J]. Infect Genet Evol, 2016, 39: 74–84. DOI: 10.1016/j.meegid.2016.01.012 |
| [11] | ANSELMO A, FLORI L, JAFFREZIC F, et al. Co-expression of host and viral microRNAs in porcine dendritic cells infected by the pseudorabies virus[J]. PLoS One, 2011, 6(3): e17374. DOI: 10.1371/journal.pone.0017374 |
| [12] | THORNBURG N J, HAYWARD S L, CROWE J E Jr. Respiratory syncytial virus regulates human microRNAs by using mechanisms involving beta interferon and NF-κB[J]. mBio, 2012, 3(6): e00220–12. |
| [13] | SLONCHAK A, HUSSAIN M, TORRES S, et al. Expression of mosquito microRNA Aae-miR-2940-5p is downregulated in response to West Nile virus infection to restrict viral replication[J]. J Virol, 2014, 88(15): 8457–8467. DOI: 10.1128/JVI.00317-14 |
| [14] | NORMAN K L, SARNOW P. Modulation of hepatitis C virus RNA abundance and the isoprenoid biosynthesis pathway by MicroRNA miR-122 involves distinct mechanisms[J]. J Virol, 2010, 84(1): 666–670. DOI: 10.1128/JVI.01156-09 |
| [15] |
李文良, 毛立, 程素平, 等. 山羊源副流感病毒3型的分离与分子鉴定[J]. 畜牧兽医学报, 2015, 46(2): 344–348.
LI W L, MAO L, CHENG S P, et al. Isolation and identification of caprine derived parainfluenza virus type 3[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(2): 344–348. (in Chinese) |
| [16] | SHRINET J, JAIN S, JAIN J, et al. Next generation sequencing reveals regulation of distinct Aedes microRNAs during chikungunya virus development[J]. PLoS Negl Trop Dis, 2014, 8(1): e2616. DOI: 10.1371/journal.pntd.0002616 |
| [17] | ZHANG Y P, JING J, LI X F, et al. Integration analysis of miRNA and mRNA expression profiles in swine testis cells infected with Japanese encephalitis virus[J]. Infect Genet Evol, 2015, 32: 342–347. DOI: 10.1016/j.meegid.2015.03.037 |
| [18] | SHRINET J, JAIN S, JAIN J, et al. Next generation sequencing reveals regulation of distinct Aedes microRNAs during chikungunya virus development[J]. PLoS Negl Trop Dis, 2014, 8(1): e2616. DOI: 10.1371/journal.pntd.0002616 |
| [19] | SUN J J, ASWATH K, SCHROEDER S G, et al. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection[J]. BMC Genomics, 2015, 16(1): 806. DOI: 10.1186/s12864-015-2044-9 |
| [20] | PEDERSEN I M, CHENG G F, WIELAND S, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism[J]. Nature, 2007, 449(7164): 919–922. DOI: 10.1038/nature06205 |
| [21] | TAGANOV K D, BOLDIN M P, CHANG K J, et al. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses[J]. Proc Natl Acad Sci U S A, 2006, 103(33): 12481–12486. DOI: 10.1073/pnas.0605298103 |
| [22] | COLLINS A S, MCCOY C E, LLOYD A T, et al. miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling[J]. PLoS One, 2013, 8(7): e69090. DOI: 10.1371/journal.pone.0069090 |
| [23] | EULALIO A, SCHULTE L, VOGEL J. The mammalian microRNA response to bacterial infections[J]. RNA Biol, 2012, 9(6): 742–750. DOI: 10.4161/rna.20018 |
| [24] | LINNSTAEDT S D, GOTTWEIN E, SKALSKY R L, et al. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus[J]. J Virol, 2010, 84(22): 11670–11678. DOI: 10.1128/JVI.01248-10 |
| [25] | LOVEDAY E K, SVINTI V, DIEDERICH S, et al. Temporal-and strain-specific host MicroRNA molecular signatures associated with swine-origin H1N1 and avian-origin H7N7 influenza A virus infection[J]. J Virol, 2012, 86(11): 6109–6122. DOI: 10.1128/JVI.06892-11 |
| [26] | FU Q, SHI H J, NI W, et al. Lentivirus-mediated Bos taurus bta-miR-29b overexpression interferes with bovine viral diarrhoea virus replication and viral infection-related autophagy by directly targeting ATG14 and ATG9A in Madin-Darby bovine kidney cells[J]. J Gen Virol, 2015, 96(Pt 1): 85–94. |
| [27] | FU Q, SHI H, SHI M, et al. bta-miR-29b attenuates apoptosis by directly targeting caspase-7 and NAIF1 and suppresses bovine viral diarrhea virus replication in MDBK cells[J]. Can J Microbiol, 2014, 60(7): 455–460. DOI: 10.1139/cjm-2014-0277 |
| [28] | DENG W W, WANG Y, LONG X P, et al. miR-21 reduces hydrogen peroxide-induced apoptosis in c-kit+ cardiac stem cells in vitro through PTEN/PI3K/Akt Signaling[J]. Oxid Med Cell Longev, 2016, 2016: 5389181. |
| [29] | WU Y R, QI H J, DENG D F, et al. MicroRNA-21 promotes cell proliferation, migration, and resistance to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal cancer[J]. Tumor Biol, 2016, 37(9): 12061–12070. DOI: 10.1007/s13277-016-5074-2 |
| [30] | HUANG J, MA G J, FU L L, et al. Pseudorabies viral replication is inhibited by a novel target of miR-21[J]. Virology, 2014, 456-457: 319–328. DOI: 10.1016/j.virol.2014.03.032 |
| [31] | DUAN L J, HAO X F, LIU Z Y, et al. MiR-129-5p is down-regulated and involved in the growth, apoptosis and migration of medullary thyroid carcinoma cells through targeting RET[J]. FEBS Lett, 2014, 588(9): 1644–1651. DOI: 10.1016/j.febslet.2014.03.002 |
| [32] | ZHANG H G, JIA R C, WANG C J, et al. Piceatannol promotes apoptosis via up-regulation of microRNA-129 expression in colorectal cancer cell lines[J]. Biochem Biophys Res Commun, 2014, 452(3): 775–781. DOI: 10.1016/j.bbrc.2014.08.150 |
| [33] | CHEN L C, CHUEH T C, TUAN Y F, et al. Activation of MAPK pathways and downstream transcription factors in 2-aminobiphenyl-induced apoptosis[J]. Environ Toxicol, 2015, 30(2): 205–211. DOI: 10.1002/tox.v30.2 |
| [34] | LIN F, PENG Y H, YANG Q H, et al. Resveratrol inhibits cadmium induced neuronal apoptosis by modulating calcium signalling pathway via regulation of MAPK/mTOR network[J]. Bangladesh J Pharmacol, 2015, 10(2): 366–376. DOI: 10.3329/bjp.v10i2.22588 |



