2. 江苏省肉羊产业工程技术研究中心, 南京 210095;
3. 启东瑞鹏牧业有限公司, 南通 226227
2. Jiangsu Engineering Technology Research Center of Mutton Sheep & Goat Industry, Nanjing 210095, China;
3. Qidong Ruipeng Animal Husbandry Co., Ltd, Nantong 226227, China
骨形态发生蛋白(Bone morphogenetic proteins, BMPs)系统在动物的生长、发育和繁殖过程中起着重要作用[1-3]。J. A.Visser与L. Gouédard等[4-5]研究指出,胚胎将与子宫产生关联时,子宫内膜间质细胞中的BMP2表达量上升,胚胎着床后的BMP2也持续表达。BMP信号通路的受体BMPR1B基因敲除的小鼠子宫形态结构遭到严重的破坏,子宫内腺体的形成受阻[6]。研究表明,BMP2、BMP4及其受体BMPR1B在小鼠发情周期的子宫中亦呈细胞特异性与阶段特异性表达[3]。
RGMb(Repulsive guidance molecule b)是排斥导向分子(RGM)家族的成员之一,广泛表达于神经系统、消化系统及生殖系统[7],作为BMP2和BMP4的辅助受体,可增强BMP信号通路的转导。S.Yura等[8]研究表明,RGMb和BMP通路的功能元件在小鼠生殖轴上共表达,可调节细胞和组织水平BMP信号转导。本课题组[9]前期试验表明,RGMb在大鼠发情周期的子宫而非卵巢中呈细胞和阶段特异性表达,同时子宫中RGMb蛋白的表达受到外源类固醇激素的影响。尽管有研究表明,定点敲除小鼠卵巢与睾丸中的RGMb基因并不影响卵巢与睾丸的机能[10],但RGMb在子宫中的作用尚不清楚。
产于中国江浙沪太湖流域的湖羊是世界著名的多胎绵羊品种。为了探讨湖羊的多胎性能,研究人员从激素分泌[11]、多胎基因[12]与子宫内环境[13]等方面进行了研究。笔者近期研究表明,对妊娠湖羊进行50%限饲处理导致羔羊宫内发育迟缓[14]。多数研究表明,限饲可影响动物的子宫和胎盘功能,引起胚胎宫内发育迟缓[15-16]。因此本研究首先分析3月龄湖羊的RGMb组织表达谱,同时分析不同发育阶段湖羊子宫中RGMb的表达定位;然后以妊娠母羊为对象,研究限饲对子宫RGMb和BMP及其受体表达的影响,为深入研究RGMb参与湖羊子宫机能调控提供试验依据。
1 材料与方法 1.1 试验动物组织表达谱试验选取体重相近的3月龄湖羊(体重为(21.01±0.30) kg)3只,屠宰后收集卵巢、垂体、下丘脑、心、脾、肾、肝、嗅脑、大脑、小脑、脑桥、子宫角、乳腺、松果体、视网膜共15种组织样品,于液氮保存。
限饲试验选取生理状态相似的18月龄湖羊30只(体重为(40.1±1.2) kg),单栏饲养,放置阴道栓(30 mg;Pharmp PTY,Herston City,Australi)诱导同期发情,人工授精(授精当日为妊娠第0天)。在妊娠第35天通过B超监测,选取怀双羔的母羊(16只)分为2组:对照组饲喂100% NRC日粮;限饲组饲喂50% NRC日粮[14]。于妊娠第110天采集子宫组织样品(n=8)。部分组织置于4%对聚甲醛中固定,部分组织置于液氮中保存。
1.2 实时荧光定量参照C.L.Meng等[17]的方法,应用Trizol(Invitrogen)提取子宫组织总RNA,利用反转录试剂盒(TaKaRa,RR036A)对总RNA进行反转录,利用定量试剂盒(TaKaRa,RR420A)进行qPCR。引物由上海英骏生物技术有限公司合成,序列见表 1。采用ΔΔCt=(Ct目的基因-Ct内参基因)法计算基因的相对表达量。
|
|
表 1 qPCR引物 Table 1 Primers used for qPCR |
参照N.N.Guo等[18]的方法对3月龄、7月龄黄体期和妊娠110 d的子宫组织制作常规石蜡切片,检测子宫组织中RGMb的表达。RGMb抗体(abcam,ab96727) 以1:200进行稀释,阴性对照采用BSA替代一抗。应用Nikon摄像机拍照分析。
1.4 蛋白免疫印迹分析参照Q.W.Wei等[19]的方法对妊娠110 d湖羊子宫组织提取总蛋白进行免疫印迹分析。RGMb与GAPDH(碧云天)抗体均以1:1 000稀释。利用Kodak Digital Sciences Image Station 440 (Eastman Kodak,Rochester,NY,USA)对目的条带显影摄像,使用Image J软件对目的条带进行分析。
1.5 数据分析应用GapdhPad5.0软件对数据进行分析。所有数据以“平均数±标准误”表示,采用t检验进行差异显著性分析,不同字母表示差异显著(P < 0.05)。
2 结果 2.1 RGMb mRNA在湖羊机体不同组织中的表达应用实时定量PCR技术检测3月龄湖羊RGMb基因在包括生殖系统在内的15种组织中的相对表达量(图 1)。以下丘脑组织中的相对表达量为1,RGMb在心、大脑和视网膜的表达量大于1,在小脑、子宫、乳腺和松果体的相对表达量介于0.5与1之间,而在剩余的垂体、卵巢等7种组织的相对表达量低于0.5,在脾中几乎不表达。
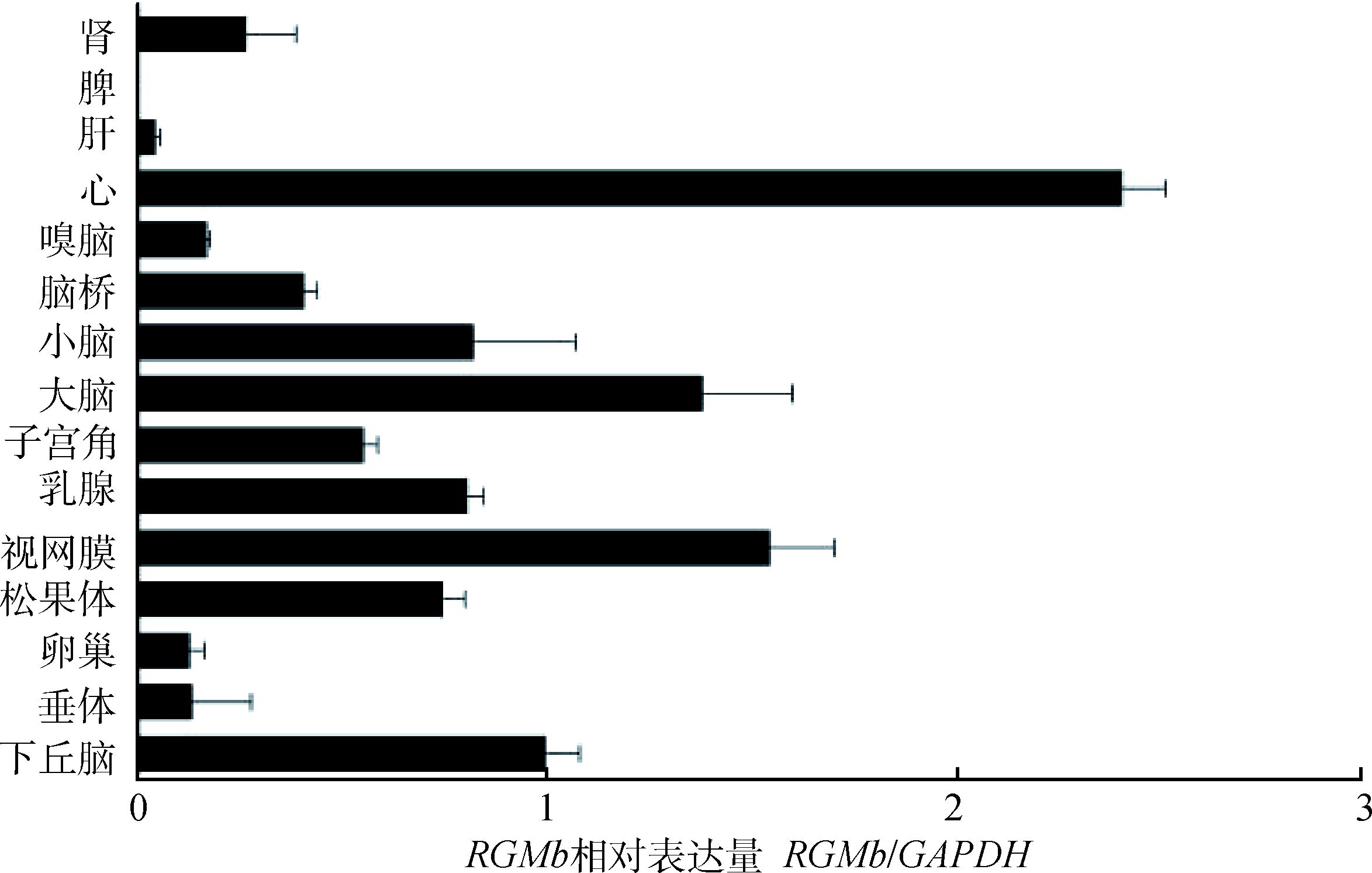
|
图 1 qPCR检测RGMb在3月龄湖羊机体组织中的表达 Figure 1 RGMb expression in different tissues of 3-month-old Hu ewes detected by qPCR |
应用免疫组织化学技术检测RGMb在性成熟前(3月龄)、性成熟后黄体期(7月龄)和妊娠期湖羊(110 d)子宫组织中的表达模式(图 2A,2B,2C)。观察不同阶段湖羊子宫形态发现,子宫内膜腺体随着年龄增加也逐渐变大,直至妊娠期达到最大。免疫组化结果显示,RGMb在3月龄(图 2A)、7月龄(图 2B)与妊娠期(图 2C)湖羊子宫的腔上皮(图 2C-g)和腺上皮(图 2C-f)细胞中均为强表达,在各个阶段湖羊子宫的环形肌层亦有弱表达(图 2C-h)。
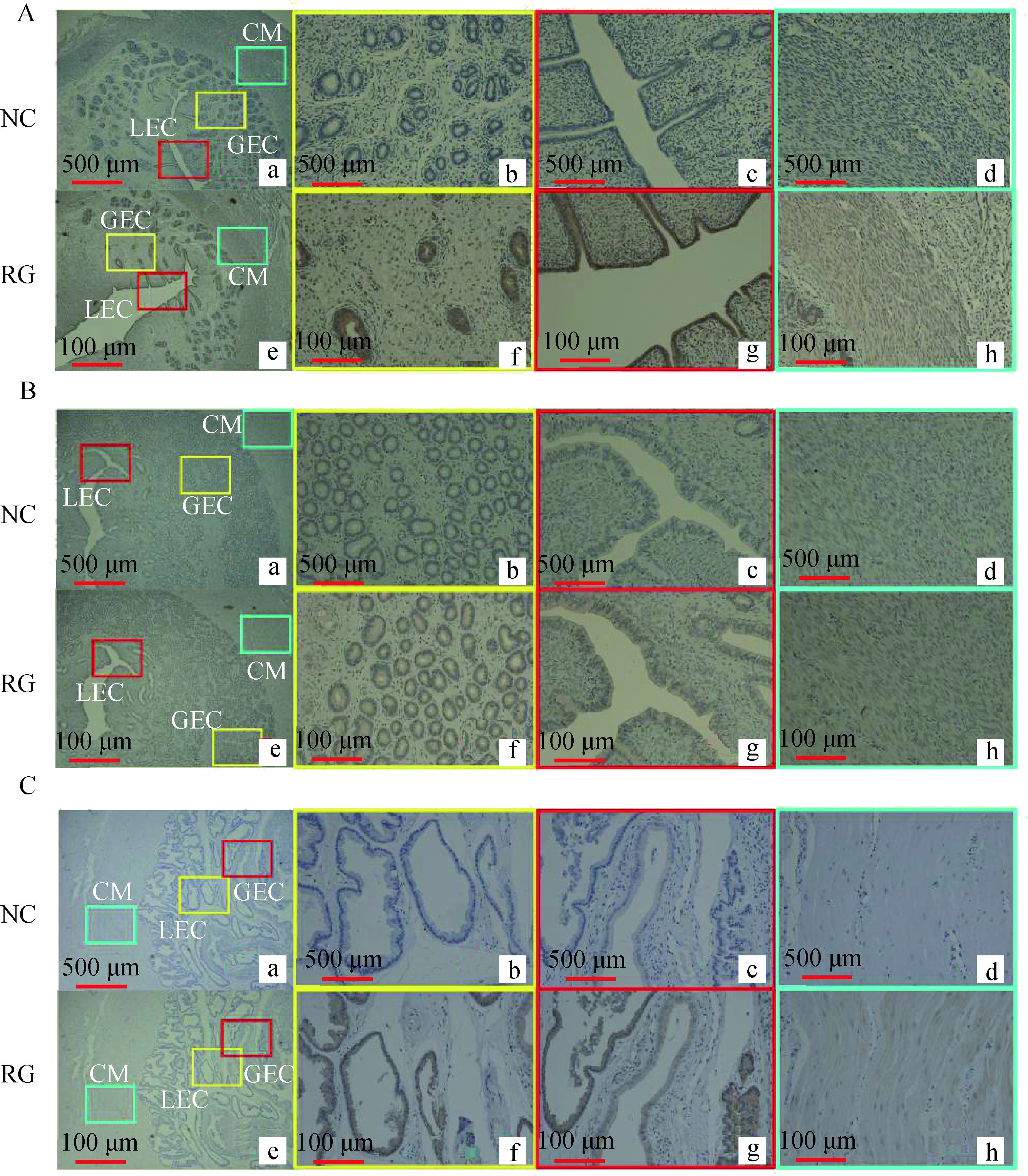
|
A.3月龄;B.7月龄黄体期;C.妊娠110 d。b,f.腺上皮(GEC);c,g.腔上皮(LEC);d,h.肌环。NC.阴性对照; RG. RGMb A. 3-month-old; B.Luteal phase of 7-month-old; C. Pregnant D110.b, f.Glandular epithelial cells; c, g. Luminal epithelial cells; d, h. Circle muscle. NC. Negative control; RG. RGMb 图 2 RGMb在3月龄、7月龄及妊娠时期湖羊子宫中的定位 Figure 2 Localization of RGMb protein in the uterus of 3-month-old, 7-month-old and pregnant Hu ewes |
应用实时定量PCR与WB技术检测限饲对妊娠湖羊子宫组织RGMb mRNA与蛋白水平的影响。结果发现,限饲对RGMb mRNA相对表达量无显著影响(P>0.05,图 3A),但限饲显著降低了RGMb蛋白的表达量(P < 0.05,图 3B)。
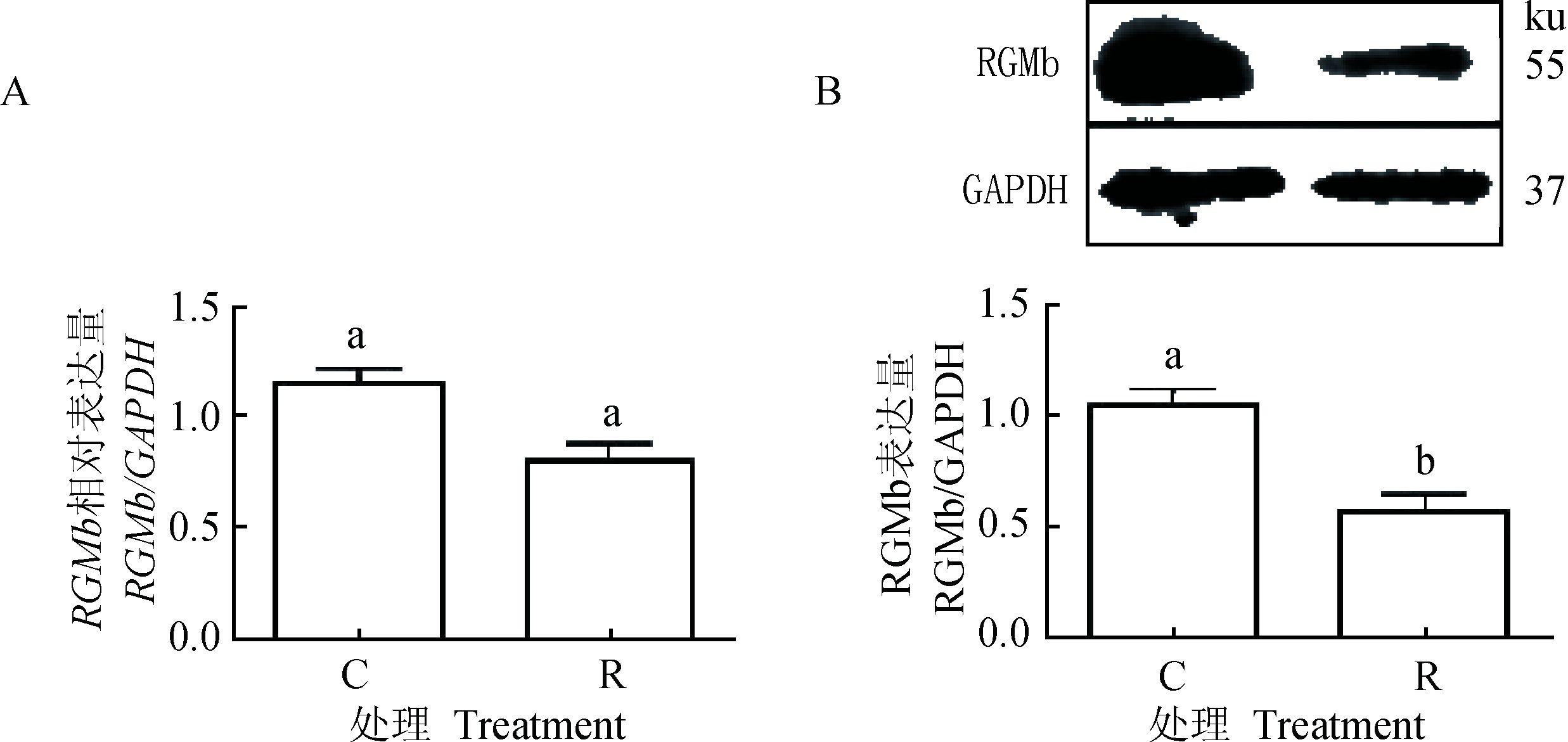
|
A.RGMb mRNA表达量;B. RGMb蛋白表达量。不同字母表示差异显著(P < 0.05),下同 A. Relative expression of RGMb mRNA; B. Relative expression of RGMb protein. Bars with different letters are significantly different (P < 0.05), the same as below 图 3 限饲对妊娠湖羊子宫RGMb蛋白和mRNA表达的影响 Figure 3 Effect of food restriction on uterine expression of RGMb protein and mRNA in pregnant Hu ewes |
BMP2与BMP4是BMP系统中的重要成员,参与调控动物生长发育过程,且在维持雌性动物子宫稳态中发挥着重要的作用。应用实时定量PCR技术检测湖羊子宫组织BMP2和BMP4 mRNA的表达。结果显示,与对照组相比,限饲组中BMP2与BMP4 mRNA表达量显著上升(P < 0.05,图 4A,4B)。
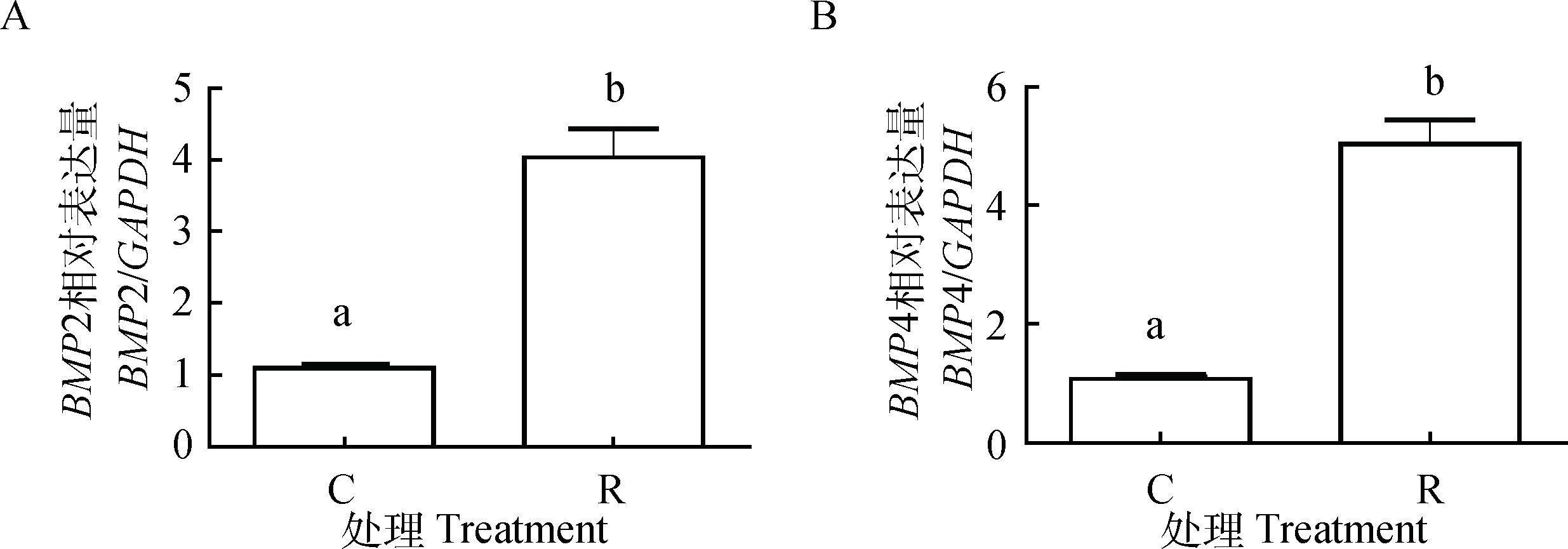
|
A.BMP2相对表达量;B.BMP4相对表达量 A. Relative expression of BMP2; B. Relative expression of BMP4 图 4 限饲对妊娠湖羊子宫BMP2和BMP4 mRNA表达的影响 Figure 4 Effect of food restriction on uterine expression of BMP2 and BMP4 mRNA in pregnant Hu ewes |
BMP受体可分为Ⅰ类与Ⅱ类受体,BMPR1A与BMPR1B属于Ⅰ类受体,BMPR2属于Ⅱ类受体。应用实时定量PCR技术检测湖羊子宫组织受体BMPR1A、BMPR1B和BMPR2 mRNA的表达。与对照组相比,限饲组中的BMPR1A、BMPR1B与BMPR2 mRNA表达水平均显著降低(P < 0.05,图 5A,5B,5C)。
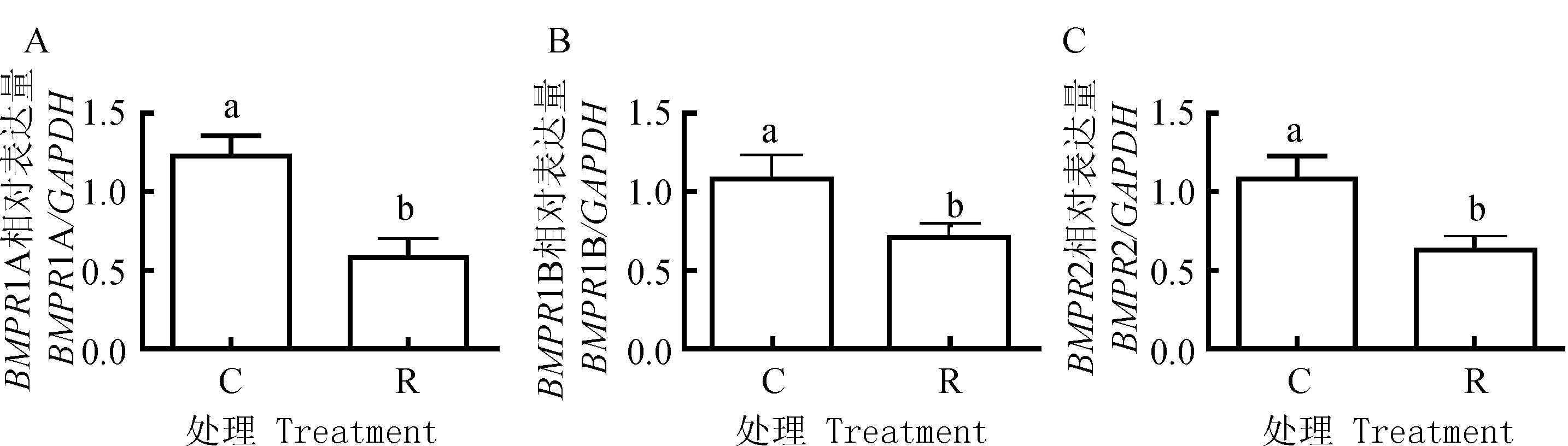
|
A.BMPR1A相对表达量;B.BMPR1B相对表达量;C.BMPR2相对表达量 A. Relative expression of BMPR1A; B. Relative expression of BMPR1B; C. Relative expression of BMPR2 图 5 限饲对妊娠湖羊子宫BMP受体mRNA表达的影响 Figure 5 Effect of food restriction on uterine mRNA expression of BMP receptors in pregnant Hu ewes |
T.A.Samad等[20]于2005年指出RGMb是BMP信号通路的辅助受体。同年,Y.Xia等[21]指出,RGMb在生殖系统的多种细胞中有表达,如睾丸生精细胞、卵巢初级卵母细胞等。本课题组对雄性湖羊的研究发现RGMb在生殖系统中表达量较高[22]。本试验以限饲湖羊为模型,探讨RGMb与BMP信号通路在湖羊子宫组织中的潜在作用。
作为BMP2与4的辅助受体,RGMb可以增强BMP的信号,促进细胞低水平配体的敏感性[23]。本研究中,RGMb在3月龄湖羊的14种不同组织(脾中未检测到)中均有表达,在心、大脑、视网膜表达量高,与已报道的RGMb在心血管、中枢神经等系统中参与重要的生理与病理过程[7, 21, 24]一致。RGMb在子宫组织中的相对表达量大于0.5,笔者前期的研究表明,RGMb在大鼠子宫有表达,且其表达量随发情周期的不同阶段而变化[17]。因此,RGMb基因可能对雌性湖羊的生殖活动具有重要的调控作用。
为了证实RGMb在湖羊子宫中的表达,我们应用IHC对不同阶段湖羊子宫中的RGMb定位进行了研究。结果发现,湖羊子宫RGMb主要定位于腔上皮和腺上皮细胞,在环形肌层有弱表达,这与先前大鼠子宫中的定位结果一致[17]。子宫RGMb在性成熟前(3月龄)、性成熟后(7月龄)与妊娠阶段(18月龄)的细胞定位模式一致,这不同于雄性小鼠RGMb在睾丸中的定位具有阶段特异性[21]。G. F. Erickson等[25]研究发现,BMP信号通路中的大多数成员均定位于子宫内膜细胞与腔上皮细胞中,如BMP4与BMP2蛋白定位于子宫内膜腔上皮、腺上皮与肌环层[3];BMP2 mRNA在小鼠子宫表达[3];BMPR1A与BMPR2B表达于子宫内膜与子宫肌膜上。RGMb与BMP信号途径功能元件的共定位提示,RGMb可能协同BMP信号通路在生殖系统方面发挥着重要的生物学作用。因此,RGMb可能参与BMP对湖羊子宫结构的维持与功能的调控。
为了研究RGMb与子宫机能之间的关系,通过对湖羊进行50%的限饲处理,结果发现,RGMb蛋白的表达水平下降,这可能是由于RGMb mRNA在湖羊子宫的表达量较高,转录后翻译的蛋白质表达量相对较低,从而导致限饲对RGMb蛋白表达量影响较大[26-27]。进一步研究发现,限饲升高了RGMb配体即BMP2与BMP4 mRNA水平,降低了BMP经典通路的受体BMPR1A、BMPR1B与BMPR2 mRNA水平,这种配体与受体相反的变化趋势,可能是由于子宫对限饲后的稳态维持反应,从而维持胎儿的生长发育与存活。大量前期研究指出,BMP信号通路通过影响细胞的增殖与凋亡进而调节动物妊娠过程[28-29]。BMP2/4可参与调控早期妊娠子宫蜕膜退化的过程[30];P.S.Tanwar等[31]指出,BMP4是维持调节子宫内膜稳态的潜在调控者。BMP受体亦是影响雌性动物生殖系统的重要因素之一。在BMPR1B基因敲除小鼠中子宫腺体的形成受阻[6];BMPR1A是胚胎植入期的关键因子[32]。RGMb与BMP信号在调控机体稳态中发挥着重要的生物学功能。转染RGMb cDNA可增强BMP的信号通路转导,但这种增强的效果具有BMP配体依赖性。RGMb可与BMP信号通路受体互作,发挥一定功能作用。研究指出,在转染RGMb的HEK293细胞中,通过免疫共沉淀技术发现,RGMb可与Ⅰ类受体(ALK2、ALK3和ALK6) 和Ⅱ类受体(ACTRIIA、BMPRII和ACTRIIB)相结合形成复合物[20]。另外,人与啮齿动物嗜酸性细胞产生的BMP2与BMP4能促进子宫环形肌与平滑肌细胞的凋亡[33-35]。本试验中,BMP配体与受体mRNA表达量出现的差异性变化,提示限饲影响了BMP信号通路系统在妊娠期湖羊子宫正常发挥功能,然而BMP信号通路与RGMb在湖羊子宫中的具体作用机制有待进一步研究。
4 结论RGMb在湖羊机体各组织中的表达为研究RGMb参与湖羊机体生长发育调控奠定了基础。限饲影响了子宫组织BMP系统成员尤其是RGMb蛋白的表达,提示RGMb可能参与湖羊子宫机能的调控,为进一步研究BMP在子宫中的作用机制提供了试验依据。
| [1] | ZHANG H, BRADLEY A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development[J]. Development, 1996, 122(10): 2977–2986. |
| [2] | TSUJI K, BANDYOPADHYAY A, HARFE B D, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing[J]. Nat Genet, 2006, 38(12): 1424–1429. DOI: 10.1038/ng1916 |
| [3] | LI Y, WEI Q W, FENG J G, et al. Expression of bone morphogenetic protein 2, 4, and related components of the BMP signaling pathway in the mouse uterus during the estrous cycle[J]. J Zhejiang Univ Sci B, 2014, 15(7): 601–610. DOI: 10.1631/jzus.B1300288 |
| [4] | VISSER J A, OLASO R, VERHOEF-POST M, et al. The serine/threonine transmembrane receptor ALK2 mediates Müllerian inhibiting substance signaling[J]. Mol Endocrinol, 2001, 15(6): 936–945. |
| [5] | GOUÉDARD L, CHEN Y G, THEVENET L, et al. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Müllerian hormone and its type Ⅱ receptor[J]. J Biol Chem, 2000, 275(36): 27973–27978. |
| [6] | YI S E, LAPOLT P S, YOON B S, et al. The type Ⅰ BMP receptor BMPRIB is essential for female reproductive function[J]. Proc Natl Acad Sci U S A, 2001, 98(14): 7994–7999. DOI: 10.1073/pnas.141002798 |
| [7] |
柳江枫, 杨宝学. RGMb/DRAGON介导的信号通路及其研究进展[J]. 神经药理学报, 2014, 4(2): 46–54.
LIU J F, YANG B X. RGMb/DRAGON mediated signaling pathways[J]. Acta Neuropharmacologica, 2014, 4(2): 46–54. (in Chinese) |
| [8] | YURA S, ITOH H, SAGAWA N, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition[J]. Cell Metab, 2005, 1(6): 371–378. DOI: 10.1016/j.cmet.2005.05.005 |
| [9] |
孟晨玲. RGMb基因在大鼠发情周期子宫和卵巢及湖羊组织中的表达研究[D]. 南京: 南京农业大学, 2014.
MENG C L. Expression patterns of RGMb in rat uterus and ovary during the estrous cycle and its expression in Hu sheep[D]. Nanjing: Nanjing Agricultural University, 2014. (in Chinese) (in Chinese) |
| [10] | MENG C L, LIU W, HUANG H, et al. Repulsive guidance molecule b (RGMb) is dispensable for normal gonadal function in mice[J]. Biol Reprod, 2016, 94(4): 78. |
| [11] |
石国庆, 茆达干, 程瑞禾, 等. 湖羊和新疆细毛羊妊娠早期内分泌比较[J]. 南京农业大学学报, 2008, 31(1): 146–148.
SHI G Q, MAO D G, CHENG R H, et al. Comparison of endocrinology during early pregnancy of Hu sheep and Xinjiang fine wool sheep[J]. Journal of Nanjing Agricultural University, 2008, 31(1): 146–148. (in Chinese) |
| [12] |
管峰, 艾君涛, 刘守仁, 等. BMPR-IB和BMP15基因作为湖羊多胎性候选基因的研究[J]. 家畜生态学报, 2005, 26(3): 9–12.
GUAN F, AI J T, LIU S R, et al. Study of BMPR-IB and BMP15 as candidate genes for prolificacy in Hu sheep[J]. Acta Ecologiae Animalis Domastici, 2005, 26(3): 9–12. (in Chinese) |
| [13] |
茆达干, 石国庆, 张红琳, 等. 湖羊早期妊娠的子宫内环境研究[J]. 畜牧兽医学报, 2010, 41(9): 1203–1207.
MAO D G, SHI G Q, ZHANG H L, et al. Study on intrauterine environment during early pregnancy of Hu sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(9): 1203–1207. (in Chinese) |
| [14] | ZHANG H, SUN L W, WANG Z Y, et al. Dietary N-carbamylglutamate and rumen-protected-arginine supplementation ameliorate fetal growth restriction in undernourished ewes[J]. J Anim Sci, 2016, 94(5): 2072–2085. DOI: 10.2527/jas.2015-9587 |
| [15] | LANGLEY-EVANS S C, DANIEL Z C, WELLS C A, et al. Protein restriction in the pregnant mouse modifies fetal growth and pulmonary development: role of fetal exposure to β-hydroxybutyrate[J]. Exp Physiol, 2011, 96(2): 203–215. DOI: 10.1113/expphysiol.2010.054460 |
| [16] | WU G Y, BAZER F W, SATTERFIELDET M C, et al. Impacts of arginine nutrition on embryonic and fetal development in mammals[J]. Amino Acids, 2013, 45(2): 241–256. DOI: 10.1007/s00726-013-1515-z |
| [17] | MENG C L, GUO N N, WEI Q W, et al. Expression of repulsive guidance molecule b (RGMb) in the uterus and ovary during the estrous cycle in rats[J]. Acta Histochem, 2014, 116(8): 1231–1236. DOI: 10.1016/j.acthis.2014.07.006 |
| [18] | GUO N N, MENG C L, BAI W J, et al. Prostaglandin F2α induces expression of activating transcription factor 3 (ATF3) and activates MAPK signaling in the rat corpus luteum[J]. Acta Histochem, 2015, 117(2): 211–218. DOI: 10.1016/j.acthis.2014.12.008 |
| [19] | WEI Q W, SHI F X. Cleavage of poly (ADP-ribose) polymerase-1 is involved in the process of porcine ovarian follicular atresia[J]. Anim Reprod Sci, 2013, 138(3-4): 282–291. DOI: 10.1016/j.anireprosci.2013.02.025 |
| [20] | SAMAD T A, REBBAPRAGADA A, BELL E, et al. DRAGON, a bone morphogenetic protein co-receptor[J]. J Biol Chem, 2005, 280(14): 14122–14129. DOI: 10.1074/jbc.M410034200 |
| [21] | XIA Y, SIDIS Y, MUKHERJEE A, et al. Localization and action of Dragon (repulsive guidance molecule b), a novel bone morphogenetic protein coreceptor, throughout the reproductive axis[J]. Endocrinology, 2005, 146(8): 3614–3621. DOI: 10.1210/en.2004-1676 |
| [22] |
孟晨玲, 王亚磊, 郭南南, 等. 湖羊RGMb基因的克隆、序列分析及表达[J]. 畜牧与兽医, 2014, 46(6): 59–62.
MENG C L, WANG Y L, GUO N N, et al. Cloning, sequence analysis and expression of RGMb gene from Hu sheep[J]. Animal Husbandry & Veterinary Medicine, 2014, 46(6): 59–62. (in Chinese) |
| [23] | MA C H, BRENNER G J, OMURA T, et al. The BMP coreceptor RGMb promotes while the endogenous BMP antagonist noggin reduces neurite outgrowth and peripheral nerve regeneration by modulating BMP signaling[J]. J Neurosci, 2011, 31(50): 18391–18400. DOI: 10.1523/JNEUROSCI.4550-11.2011 |
| [24] | XIAO Y P, YU S H, ZHU B G, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance[J]. J Exp Med, 2014, 211(5): 943–959. DOI: 10.1084/jem.20130790 |
| [25] | ERICKSON G F, FUQUA L, SHIMASAKI S. Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle[J]. Endocrinology, 2004, 182(2): 203–217. DOI: 10.1677/joe.0.1820203 |
| [26] |
胡兰.
动物生物化学[M]. 北京: 中国农业大学出版社, 2007.
HU L. Animal biochemistry[M]. Beijing: China Agricultural University Press, 2007. (in Chinese) |
| [27] | GU L B, ZHU N X, ZHANG H Y, et al. Regulation of XIAP translation and induction by MDM2 following irradiation[J]. Cancer Cell, 2009, 15(5): 363–375. DOI: 10.1016/j.ccr.2009.03.002 |
| [28] | LIU G, LIN H, ZHANG X, et al. Expression of Smad2 and Smad4 in mouse uterus during the oestrous cycle and early pregnancy[J]. Placenta, 2004, 25(6): 530–537. DOI: 10.1016/j.placenta.2003.11.006 |
| [29] | PARIA B C, DAS S K, ANDREWS G K, et al. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation[J]. Proc Natl Acad Sci U S A, 1993, 90(1): 55–59. DOI: 10.1073/pnas.90.1.55 |
| [30] | SHIMASAKI S, MOORE R K, OTSUKA F, et al. The bone morphogenetic protein system in mammalian reproduction[J]. Endocr Rev, 2004, 25(1): 72–101. DOI: 10.1210/er.2003-0007 |
| [31] | TANWAR P S, MCFARLANE J R. Dynamic expression of bone morphogenetic protein 4 in reproductive organs of female mice[J]. Reproduction, 2011, 142(4): 573–579. DOI: 10.1530/REP-10-0299 |
| [32] | MONSIVAIS D, CLEMENTI C, PENG J, et al. Uterine ALK3 is essential during the window of implantation[J]. Proc Natl Acad Sci U S A, 2016, 113(3): E387–E395. DOI: 10.1073/pnas.1523758113 |
| [33] | LEE M J, YANG C W, JIN D C, et al. Bone morphogenetic protein-7 inhibits constitutive and interleukin-1β-induced monocyte chemoattractant protein-1 expression in human mesangial cells: role for JNK/AP-1 pathway[J]. J Immunol, 2003, 170(5): 2557–2563. DOI: 10.4049/jimmunol.170.5.2557 |
| [34] | MIYAZAKI Y, OSHIMA K, FOGO A, et al. Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development[J]. Kidney Int, 2003, 63(3): 835–844. DOI: 10.1046/j.1523-1755.2003.00834.x |
| [35] | WILLETTE R N, GU J L, LYSKO P G, et al. BMP-2 gene expression and effects on human vascular smooth muscle cells[J]. J Vasc Res, 1999, 36(2): 120–125. DOI: 10.1159/000025634 |



