牛乳脂肪主要由乳腺上皮细胞(Bovine mammary gland epithelial cells, BMECs)以脂肪球的形式分泌,乳脂的合成分泌受多种激素和细胞因子的调节[1]。胰岛素样生长因子-Ⅰ(Insulin like growth factors-1, IGF-1) 是调节BMECs生长发育及泌乳功能的重要细胞因子[2-3],而脐带间充质干细胞(Umbilical cord mesenchymal stem cells, UC-MSCs)可以分泌IGF-1在内的多种细胞因子,这些细胞因子能够作用于周围细胞发挥旁分泌作用[4-5]。多数研究通过灌注[6]、体外单一添加[7]IGF-1或与其他物质组合[8]探究其对乳腺上皮细胞增殖、分化及乳腺泌乳性能的作用,但对BMECs乳脂合成的报道甚少。本课题组前期已成功构建UC-MSCs和BMECs无血清最佳共培养条件,且UC-MSCs可以提高BMECs的IGF-1和IGF-1R的分泌水平[9],在此基础上,将UC-MSCs和BMECs共培养观测非外源性IGF-1对BMECs乳脂合成的影响,以弥补BMECs自身不能持续分泌细胞因子的问题,为细胞水平上研究乳脂合成提供新的思路。
1 材料与方法 1.1 试验材料 1.1.1 试验细胞来源UC-MSCs:前期试验体外分离培养并鉴定的荷斯坦奶牛脐带间充质干细胞;BMECs:购自广州吉妮欧生物科技有限公司。
1.1.2 主要仪器和试剂倒置显微镜(Motic-AE31,日本),CO2培养箱(HF151UV,中国),TranswellTM小室(Corning,美国),AG1024(Alexis,德国),AG490(Sigma,美国),Elisa试剂盒(上海博谷生物科技有限公司),甘油三酯试剂盒(南京建成,F001-1),MX3000P实时荧光定量PCR(Real-time quantitative PCR,RT-qPCR)仪(Agilent, Germany),细胞总RNA提取试剂盒(LN-0108A,上海诺伦生物医药技术有限公司)。
1.2 试验设计与分组在前期试验基础上,按照UC-MSCs和BMECs共培养最佳浓度比(1:2) 和时间(48 h),共培养组应用TranswellTM小室(孔径0.4 μm)建立上下双层细胞的共培养体系,在上室接种1 mL BMECs 1×105·孔-1,下室接种2 mL UC-MSCs1×105·孔-1,共培养组48 h后分别给予IGF-1R抑制剂AG1024(10 μmol·L-1)、JAK/STAT信号阻断剂AG490(50 μmol·L-1)处理24 h,吸取上清,并采用胰酶消化法收集细胞,-20 ℃保存备用。均置于37 ℃、5% CO2培养箱常规培养,培养液均为无血清基础培养基(SFM)。每组均为3个平行处理。试验分组详见表 1。
|
|
表 1 不同处理及分组 Table 1 Different treatments and groups |
采用双抗体夹心法测定各组上清中IGF-1浓度水平,方法按照ELISA试剂盒(上海博谷生物科技有限公司)说明书进行;TG含量按照甘油三酯试剂盒(南京建成,F001-1) 进行测定。
1.3.2 实时荧光定量PCR参照试剂盒说明书提取各组细胞总RNA,全自动酶联免疫分析仪测出RNA浓度以及OD260 nm/OD280 nm,以提取的总RNA为模板,按照逆转录试剂盒说明书(10 μL,37 ℃ 15 min, 85 ℃ 5 s)合成cDNA。PCR按20 μL反应体系进行,反应条件: 95 ℃ 30 s变性,95 ℃ 5 s,60 ℃ 31 s,50个循环,上机自动分析荧光信号,并将其转换Ct值,采用ΔΔCt法分析,以磷酸甘油醛脱氢酶(GAPDH)为内参基因计算ACACA、FASN、SREBP 1 mRNA相对定量值(2-ΔΔCt)。基因序列均从GenBank中获取,引物用Primer5.0软件进行设计,由上海博古生物科技有限公司合成,详见表 2。
|
|
表 2 引物序列 Table 2 Sequences of primer |
数据用SPSS 18.0软件进行ANOVA方差分析,采用Duncan氏方法对各组间平均数进行多重比较,结果表示为“平均值±标准误(x±S)”,P < 0.05表示差异显著。
2 结果 2.1 RT-qPCR鉴定样本浓度见表 3,样本RNA浓度保持在200~400 ng·μL-1,OD260 nm/OD280 nm均为1.8~2.2,表明总RNA纯度较高,可用于后续试验。
|
|
表 3 各组总RNA浓度和纯度 Table 3 Total RNA concentration and purity of each group |
各基因扩增图谱和熔解曲线见图 1,扩增图谱显示每个基因的Ct值,Ct值是反应体系中荧光信号达到阀值时经历的循环数,熔解曲线出现为单一平滑峰,基线平稳,表明PCR产物单一,能准确定量目的基因mRNA的表达量。
|
A, B.GAPDH; C, D.ACACA; E, F.FASN; G, H.SREBP1 图 1 各基因扩增图谱(A、C、E、G)和熔解曲线(B、D、F、H) Figure 1 Amplification plots (A, C, E, G) and dissolution curves (B, D, F, H) of each gene |
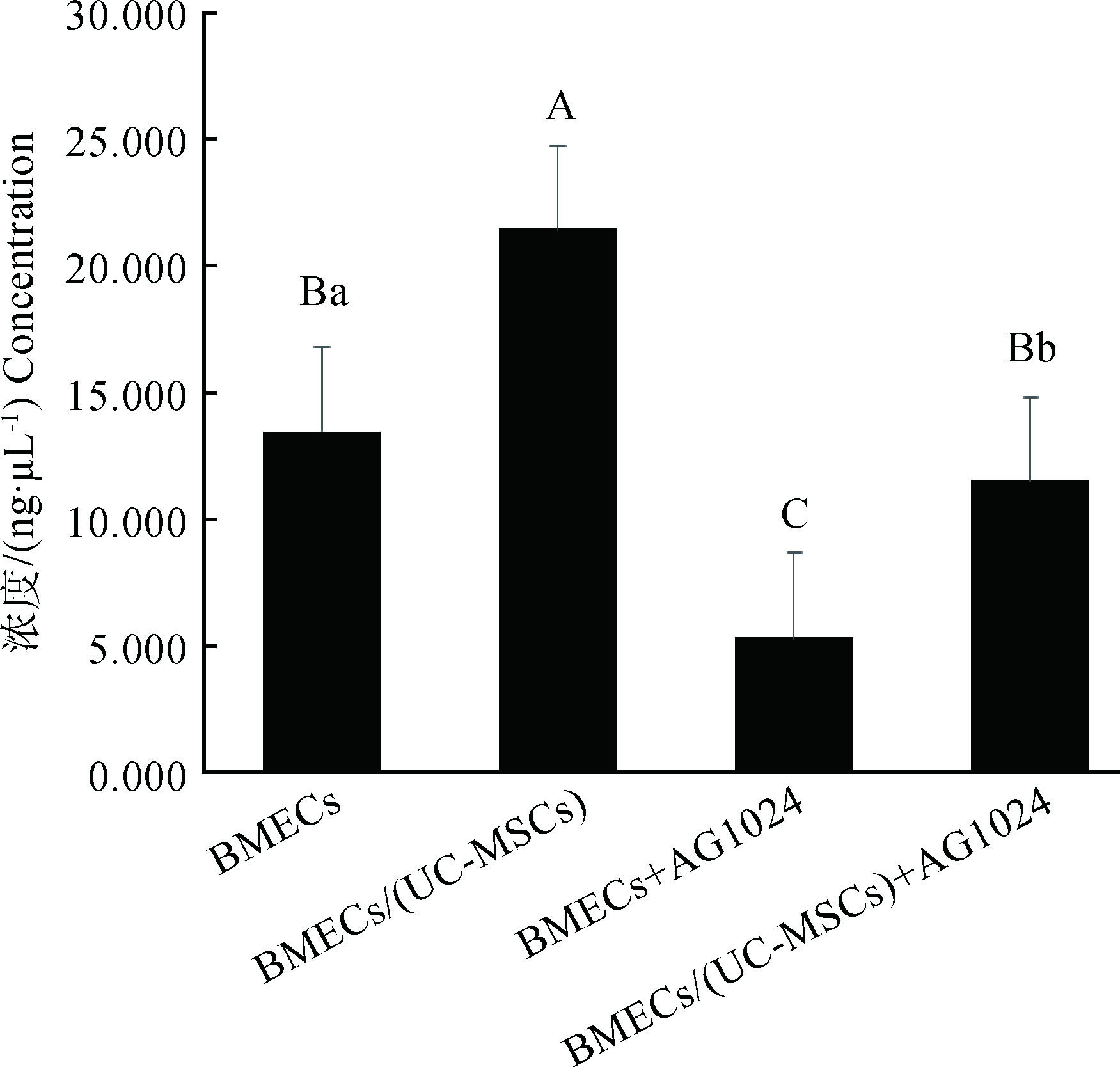
如图 2所示,共培养组IGF-1含量极显著高于对照组(P < 0.01);BMECs+AG1024组IGF-1含量极显著低于对照组(P < 0.01),BMECs/(UC-MSCs)+AG1024组IGF-1含量极显著低于BMECs/(UC-MSCs)组(P < 0.01),BMECs/(UC-MSCs)+AG1024组极显著高于BMECs+AG1024组(P < 0.01)。
|
柱形图上方相同字母间表示差异不显著(P>0.05),不同小写字母间表示差异显著(P < 0.05),不同大写字母间表示差异极显著(P < 0.01)。下同 Above the column chart values with the same letter mean no significant difference(P>0.05), the different small letter mean significant difference(P < 0.05), and the different capital letter mean extremely significant difference(P < 0.01).The same as below 图 2 AG1024对BMECs中IGF-1含量的影响 Figure 2 Effect of AG1024 on the content of IGF-1 in BMECs |
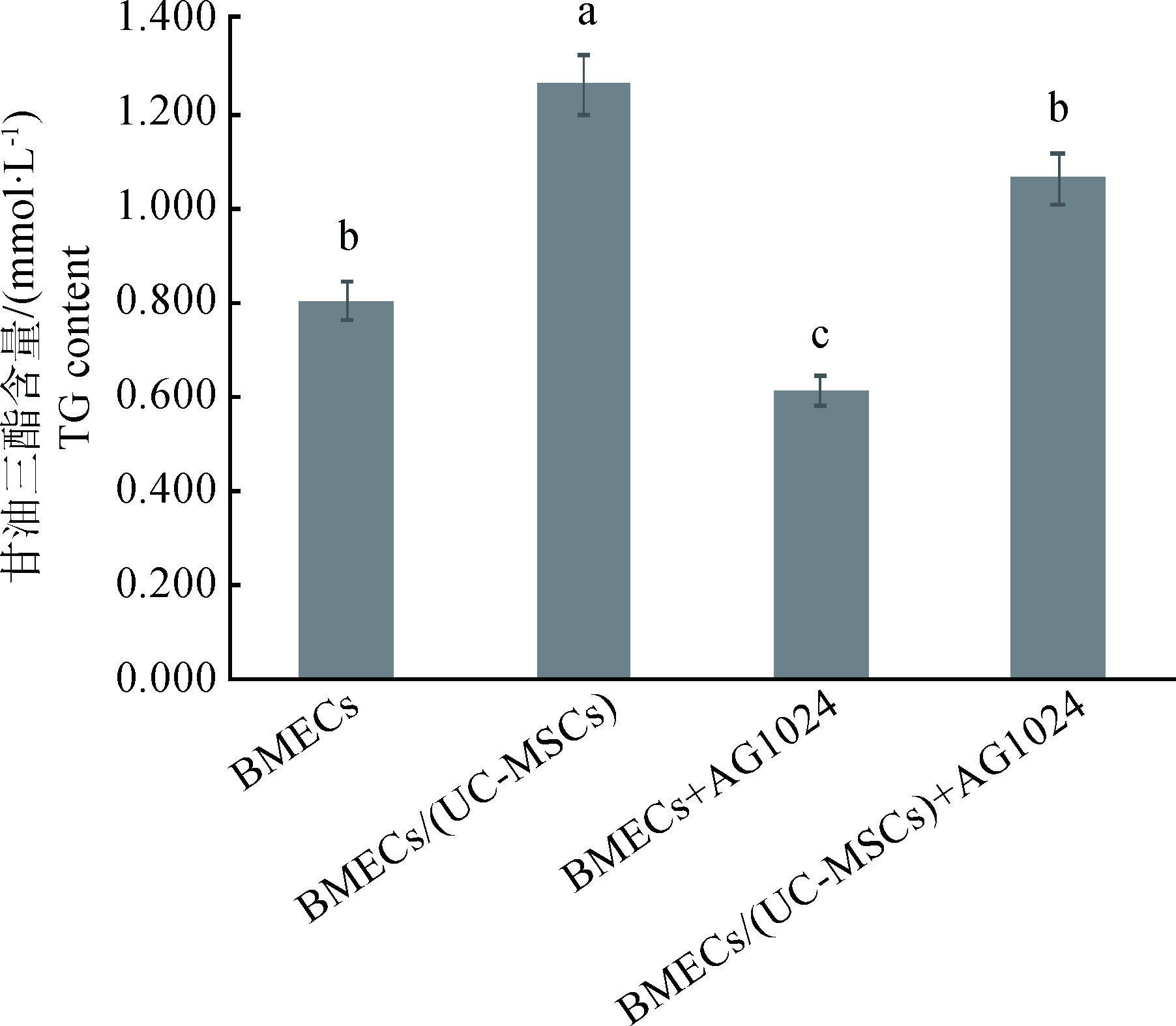
如图 3所示,共培养组TG含量显著高于对照组(P < 0.05);BMECs+AG1024组TG含量显著低于对照组(P < 0.05);BMECs/(UC-MSCs)+AG1024组TG含量显著低于共培养组(P < 0.05),显著高于BMECs+AG1024组(P < 0.05)。
|
图 3 AG1024对BMECs中TG含量的影响 Figure 3 Effect of AG1024 on the content of TG in BMECs |
结果如表 4所示,与对照组相比,共培养组ACACA、FASN、SREBP 1 mRNA的相对表达丰度显著升高(P < 0.05),BMECs+AG1024、BMECs+AG490+AG1024组显著降低(P < 0.05),BMECs+AG490组则差异不显著(P>0.05);与共培养组ACACA、FASN、SREBP 1 mRNA的相对表达丰度相比,BMECs/(UC-MSCs)+AG1024、BMECs/(UC-MSCs)+AG490+AG1024组均显著降低(P < 0.05),BMECs/(UC-MSCs)+AG490组无显著性差异(P>0.05);BMECs+AG490+AG1024组较BMECs+AG1024相比3种基因相对表达量均无显著差异(P>0.05);处理的共培养组组相对表达丰度均显著高于处理的对照组(P < 0.05)。
|
|
表 4 AG490对BMECs乳脂合成关键基因表达的影响 Table 4 Effect of AG490 on key genes expression of BMECs milk fat synthesis |
奶牛乳脂肪中的主要成分是甘油三酯(TG),占乳脂的96%~98%,是由脂肪酸和α-磷酸甘油在乳腺上皮细胞中合成[10-11];ACACA是催化脂肪酸合成反应的限速酶,FASN是乳腺内脂肪合成途径中的一个相关酶,FASN基因的表达直接影响着脂肪酸合成酶的多寡,而SREBP基因的高表达可导致脂肪合成相关酶基因的高表达[12-13],因此奶牛乳腺乳脂合成及运输中需要ACACA、FASN、SREBP等多种基因的协调表达。
本研究图 3结果显示,与UC-MSCs共培养之后,BMECs中TG含量显著升高,表 4中可以看出,共培养之后BMECs中ACACA、FASN、SREBP 1 mRNA的相对表达丰度显著也显著升高。说明UC-MSCs可以上调UC-MSCs中ACACA、FASN、SREBP 1 mRNA的表达,促进BMECs中TG的分泌量增加,这可能与UC-MSCs的自分泌或旁分泌作用有关。IGF-1作为一种重要的乳腺发育及泌乳的调控因子,与IGF-1R结合调控奶牛乳腺发育及泌乳功能[14],UC-MSCs可以分泌IGF-1在内的多种细胞因子[15-16],因此本试验利用IGF-1R抑制剂来探究UC-MSCs分泌的IGF-1是否参与对BMECs乳脂合成的调节作用。结果表明,与UC-MSCs共培养极显著提高了BMECs中IGF-1含量,加入AG1024后对IGF-1具有明显的抑制作用;从图 3可以看出,IGF-1被抑制后,BMECs中的TG含量显著降低,但此时与UC-MSCs共培养的BMECs中TG含量仍显著高于单纯BMECs培养组。说明IGF-1参与UC-MSCs对BMECs中TG分泌的调节作用。从表 4数据可以看出,单独添加AG1024则显著下调了两组BMECs中ACACA、FASN、SREBP 1 mRNA的相对表达丰度,但IGF-1被抑制之后的共培养组仍显著高于BMECs组,说明IGF-1被抑制后,UC-MSCs可以继续对BMECs的乳脂合成关键基因的表达起促进作用,这也和图 3显示的TG含量变化相吻合。体外培养的BMECs自身分泌的IGF-1有限,而UC-MSCs能够持续分泌表达IGF-1,且可以作用于自身和其它组织细胞,即自分泌和旁分泌功能[17-18]。来源于UC-MSCs的IGF-1通过上调BMECs中ACACA、FASN、SREBP 1 mRNA的表达丰度,从而提高BMECs的TG含量。研究表明IGF-1能够显著促进CSN2和ACACA基因的转录[19];王皓宇等[20]通过体外添加一定浓度的IGF-1能显著提高BMECs的ACACA、FASN和FABP 3 mRNA相对表达丰度。这与本研究结果一致。SREBP 1在BMECs中参与脂肪酸从头合成,通过影响TG酯化相关酶实现对脂类合成的调控[21-22];而在调控乳脂合成的基因网络中,ACACA和FASN是参与奶牛乳脂肪酸从头合成过程的2个关键基因[23];IGF-1可增加SREBP 1的活性而促进脂肪的合成[24]。这提示与UC-MSCs共培养促进了BMECs中IGF-1含量增加,刺激BMECs中SREBP 1的表达增加,共同上调ACACA、FASN mRNA的表达丰度,从而促进BMECs的TG分泌量。
本研究利用TranswellTM小室建立上下双层共培养体系,且采用的无血清培养基排除了其他外源因子的干扰,能够准确反映UC-MSC分泌的IGF-1对BMECs的作用,UC-MSCs对BMECs中TG的分泌及乳脂合成相关酶基因的表达丰度起到一定的促进作用,这一调节作用是通过IGF-1的参与而得以实现,可能是UC-MSCs分泌的IGF-1与BMECs中IGF-1R结合,激活IGF-1介导的泌乳相关细胞信号转导途径,从而影响BMECs乳脂合成。本研究利用JAK2/STAT5信号通路阻断剂以明确IGF-1是否介导JAK2/STAT5信号通路参与调控BMECs乳脂合成。结果表明,加入AG490阻断JAK2/STAT5信号通路后,BMECs中ACACA、FASN、SREBP 1 mRNA的相对表达丰度并无显著变化,而IGF-1和JAK2/STAT5信号通路同时被抑制时,BMECs中这3种基因的表达与仅抑制IGF-1时相比变化无显著性差异。说明JAK2/STAT5被阻断并不影响BMECs中乳脂合成关键基因的表达,IGF-1不能直接介导JAK2/STAT5信号通路调控BMECs的乳脂合成。由催乳素介导的JAK2/STAT5信号通路调控乳腺发育及乳脂、乳蛋白合成[25-26];Y.L.Huang等[27]称JAK2/STAT5信号通路可能通过调节SREBP 1 c的表达来参与BMECs中乳脂合成的调控。这和本研究结果不同,可能是培养时间、培养方式、干扰因子等不同,得出的试验结果也不相同,具体原因还有待进一步深入探究。本研究结果表明,ACACA、FASN、SREBP 1作为BMECs乳脂合成调控网络中的关键因子,受到来自UC-MSCs中的IGF-1的调节,而乳脂合成这些关键基因的表达与JAK2/STAT5信号通路无明显相关关系。
乳脂合成关键基因的表达受多条信号通路的调控[28-29],但本研究中,JAK2/STAT5信号通路被阻断后,共培养的BMECs乳脂合成关键基因仍高于同样被阻断的单纯BMECs培养组,可能IGF-1通过激活其他信号通路实现对靶基因的调控,从而促进BMECs乳脂的合成。JAK2/STAT5信号传导通路主要调控乳蛋白基因的表达,PI3K/AKT/mTOR信号传导通路主要调节乳脂肪的合成[30]。由此猜测UC-MSCs中IGF-1对BMECs乳脂合成的调节作用不是JAK2/STAT5信号通路参与介导的,可能跟PI3K/AKT/mTOR信号通路有关,但本试验未设计阻断PI3K/AKT/mTOR信号通路对BMECs乳脂合成的影响,有待于进一步深入研究。
| [1] | ACCORNERO P, MARTIGNANI E, MIRETTI S, et al. Epidermal growth factor and hepatocyte growth factor receptors collaborate to induce multiple biological responses in bovine mammary epithelial cells[J]. J Dairy Sci, 2009, 92(8): 3667–3675. DOI: 10.3168/jds.2008-1835 |
| [2] | MARSHMAN E, STREULI C H. Insulin-like growth factors and insulin-like growth factor binding proteins in mammary gland function[J]. Breast Cancer Res, 2002, 4(6): 231–239. DOI: 10.1186/bcr535 |
| [3] | SHUSHANOV S S. Insulin-like growth factors 1 and 2 regulate expression of β-casein in vitro in mouse mammary epithelial cells[J]. Bull Exp Biol Med, 2011, 152(2): 202–205. DOI: 10.1007/s10517-011-1488-4 |
| [4] | CHEN X G, KATAKOWSKI M, LI Y, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production[J]. J Neurosci Res, 2002, 69(5): 687–691. DOI: 10.1002/(ISSN)1097-4547 |
| [5] | SHOICHET M S. Polymer scaffolds for biomaterials applications[J]. Macromolecules, 2010, 43(2): 581–591. DOI: 10.1021/ma901530r |
| [6] | GAJEWSKA M, ZIELNIOK K, DEBSKI B, et al. IGF-Ⅰ retards proper development of acinar structures formed by bovine mammary epithelial cells via sustained activation of Akt kinase[J]. Domest Anim Endocrinol, 2013, 45(3): 111–121. DOI: 10.1016/j.domaniend.2013.06.005 |
| [7] |
季昀. GH和IGF-Ⅰ对奶牛乳腺上皮细胞中酪蛋白合成的调节作用机制[D]. 扬州: 扬州大学, 2013.
JI J. Mechanism and regulation of GH and IGF-Ⅰ on casein synthesis in bovine mammary epithelial cells[D]. Yangzhou: Yangzhou University, 2013. (in Chinese) (in Chinese) |
| [8] |
李莹莹, 夏小静, 吴云娣, 等. 胰岛素样生长因子-Ⅰ、肝细胞生长因子、转化生长因子-β1、干扰素-γ对奶牛乳腺上皮细胞增殖活性的影响[J]. 中国畜牧兽医, 2014, 41(4): 37–42.
LI Y Y, XIA X J, WU Y D, et al. Effects of IGF-Ⅰ, HGF, TGF-β1, IFN-γ on proliferation of bovine mammary epithelial cells[J]. China Animal Husbandry & Veterinary Medicine, 2014, 41(4): 37–42. (in Chinese) |
| [9] |
王立文. 奶牛乳腺上皮细胞和奶牛脐带间充质干细胞共培养的试验研究[D]. 乌鲁木齐: 新疆农业大学, 2014.
WANG L W. Study on the co-culture of bovine mammary epithelial cells with bovine umbilical cord mesenchymal stem cells in vitro[D]. Urumchi: Xinjiang Agricultural University, 2014. (in Chinese) (in Chinese) |
| [10] | JENSEN R G. The composition of bovine milk lipids: January 1995 to December 2000[J]. J Dairy Sci, 2002, 85(2): 295–350. DOI: 10.3168/jds.S0022-0302(02)74079-4 |
| [11] |
胡宝森. 影响奶牛乳脂合成的因素分析[J]. 山东畜牧兽医, 2010, 31(12): 71–72.
HU B S. Analysis of factors affecting milk fat synthesis in dairy cows[J]. Shandong Journal of Animal Science and Veterinary Medicine, 2010, 31(12): 71–72. DOI: 10.3969/j.issn.1007-1733.2010.12.044 (in Chinese) |
| [12] |
胡菡, 王加启, 李发弟, 等. 奶牛乳腺脂肪酸合成相关基因研究进展[J]. 生物技术通报, 2009(10): 34–39.
HU H, WANG J Q, LI F D, et al. Advance of fatty acid synthesis regulated genes from dairy cow[J]. Biotechnology Bulletin, 2009(10): 34–39. (in Chinese) |
| [13] | HORTON J D, GOLDSTEIN J L, BROWN M S. SREBPs: transcriptional mediators of lipid homeostasis[J]. Cold Spring Harb Symp Quant Biol, 2002, 67: 491–498. DOI: 10.1101/sqb.2002.67.491 |
| [14] |
崔英俊, 李庆章. 奶牛不同发育时期乳腺组织IGF-Ⅰ及其受体的表达与定位[J]. 中国乳品工业, 2014, 42(6): 4–6, 15.
CUI Y J, LI Q Z. Expressions and localizations of IGF-Ⅰ and it's receptor at different developmental stages in dairy cow mammary gland[J]. China Dairy Industry, 2014, 42(6): 4–6, 15. (in Chinese) |
| [15] | TAKAHASHI M, LI T S, SUZUKI R, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury[J]. Am J Physiol Heart Circ Physiol, 2006, 291(2): H886–H893. DOI: 10.1152/ajpheart.00142.2006 |
| [16] |
王黎明. 脐带间充质干细胞的分泌蛋白表达谱研究[D]. 合肥: 安徽医科大学, 2014.
WANG L M. The research for proteins expression profile of umbilical cord mesenchymal stem cells[D]. Hefei: Anhui Medical University, 2014. (in Chinese) (in Chinese) |
| [17] |
刘秀华, 唐朝枢. 干细胞的旁分泌和自分泌功能--基础与临床研究的新视角[J]. 生理科学进展, 2008, 39(3): 196–202.
LIU X H, TANG C S. The paracrine and autocrine of stem cells: a new frontier of basic and clinical research[J]. Progress in Physiological Sciences, 2008, 39(3): 196–202. (in Chinese) |
| [18] | SADAT S, GEHMERT S, SONG Y H, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-Ⅰ and VEGF[J]. Biochem Biophys Res Commun, 2007, 363(3): 674–679. DOI: 10.1016/j.bbrc.2007.09.058 |
| [19] |
何伯萍, 邢艳苹, 雷连成, 等. IGF-Ⅰ对奶牛乳腺上皮细胞合成乳脂、乳蛋白的影响[J]. 中国兽医学报, 2012, 32(8): 1231–1234.
HE B P, XING Y P, LEI L C, et al. IGF-Ⅰ regulating synthesis of milk fat and milk protein in bovine mammary epi-thelial ceils[J]. Chines Journal of Veterinary Science, 2012, 32(8): 1231–1234. (in Chinese) |
| [20] |
王皓宇, 秦彤, 郝海生. 不同浓度胰岛素样生长因子-Ⅰ对体外培养奶牛乳腺上皮细胞乳蛋白和脂肪合成相关基因表达的影响[J]. 中国畜牧兽医, 2013, 40(7): 1–5.
WANG H Y, QIN T, HAO H S. Effects of different concentration insulin-like growth factor-Ⅰ on expression of the related genes of milk protein and fat synthesis in bovine mammary epithelial cells cultured in vitro[J]. China Animal Husbandry & Veterinary Medicine, 2013, 40(7): 1–5. (in Chinese) |
| [21] | MA L, CORL B A. Transcriptional regulation of lipid synthesis in bovine mammary epithelial cells by sterol regulatory element binding protein-1[J]. J Dairy Sci, 2012, 95(7): 3743–3755. DOI: 10.3168/jds.2011-5083 |
| [22] | HARVATINE K J, BAUMAN D E. SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA[J]. J Nutr, 2006, 136(10): 2468–2474. |
| [23] | LEHNE R, KUKSIS A. Biosynthesis of triacylglycerols[J]. Prog Lipid Res, 1996, 35(2): 169–201. DOI: 10.1016/0163-7827(96)00005-7 |
| [24] | SMITH T M, GILLILAND K, CLAWSON G A, et al. IGF-1 Induces SREBP-1 expression and lipogenesis in SEB-1 Sebocytes via activation of the phosphoinositide 3-Kinase/Akt Pathway[J]. J Invest Dermatol, 2008, 128(5): 1286–1293. DOI: 10.1038/sj.jid.5701155 |
| [25] | AKERS R M. Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows[J]. J Dairy Sci, 2006, 89(4): 1222–1234. DOI: 10.3168/jds.S0022-0302(06)72191-9 |
| [26] |
佟慧丽, 高学军, 李庆章, 等. 胰岛素、催乳素对奶山羊乳腺上皮细胞泌乳功能的影响[J]. 畜牧兽医学报, 2008, 39(6): 721–725.
TONG H L, GAO X J, LI Q Z, et al. Impacting of insulin and prolactin on mammary gland epithelial cell line[J]. Acta Veterinaria et Zootechnica Sinica, 2008, 39(6): 721–725. (in Chinese) |
| [27] | HUANG Y L, ZHAO F, LUO C C, et al. SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro[J]. Molecules, 2013, 18(10): 12987–13002. DOI: 10.3390/molecules181012987 |
| [28] | MCFADDEN J W, CORL B A. Activation of liver X receptor (LXR) enhances de novo fatty acid synthesis in bovine mammary epithelial cells[J]. J Dairy Sci, 2010, 93(10): 4651–4658. DOI: 10.3168/jds.2010-3202 |
| [29] | RIUS A G, APPUHAMY J A D R N, CYRIAC J, et al. Regulation of protein synthesis in mammary glands of lactating dairy cows by starch and amino acids[J]. J Dairy Sci, 2010, 93(7): 3114–3127. DOI: 10.3168/jds.2009-2743 |
| [30] |
生冉, 闫素梅. 催乳素与其他激素对乳腺内乳成分合成的协同调节作用[J]. 动物营养学报, 2014, 26(6): 1435–1443.
SHENG Y, YAN S M. Prolactin and other hormones: synergistic action on regulating milk composition synthesis in mammary glands[J]. Chinese Journal of Animal Nutrition, 2014, 26(6): 1435–1443. (in Chinese) |



