2. 甘肃省肉羊繁育生物技术工程实验室, 民勤 733300
2. Sheep Breeding Biotechnology Engineering Laboratory of Gansu Province, Minqin 733300, China
糖皮质激素是一种类固醇激素,可广泛作用于反刍动物的器官和体组织,参与糖代谢、脂代谢和蛋白质代谢过程。糖皮质激素对家畜的泌乳、生殖、心血管和中枢神经系统也有调节作用[1-2]。对奶畜而言,糖皮质激素对乳腺发育和泌乳有调控作用[3],可以降低奶产量,稳定机体能量平衡状态[4]。正常生理条件下,家畜血液中糖皮质激素水平很稳定,但应激(如断奶应激、热应激等)和炎症标志物可刺激下丘脑-垂体-肾上腺轴(HPA)引起交感神经兴奋[5],下丘脑释放促肾上腺皮质激素释放激素(CRH)和血管增压素(VP)二者协同调控肾上腺皮质分泌糖皮质激素[6-8]。糖皮质激素通过自由扩散的方式穿过细胞膜进入靶细胞,激活细胞质中的糖皮质激素受体(GR),GR进入细胞核对靶基因进行核转录调控从而影响反刍动物的免疫系统[9]。糖皮质激素可在细胞和转录水平上对家畜免疫系统产生抑制,从而控制炎症反应,起到抗炎作用;另一方面,糖皮质激素对细胞因子的调节存在双向通路,在某些情况下还有致炎作用[10]。与其他动物相比反刍动物更容易受到应激影响,尤其是幼龄反刍动物发生断奶应激时会增加其患呼吸道疾病的风险[11]。目前,有关反刍动物应激反应中糖皮质激素免疫调节作用的研究报道较少,多作为应激反应的生物学标志物,而没有对其功能进行系统研究,尤其是在分子水平以及作用机理上的研究鲜有报道。本文就反刍动物应激反应中糖皮质激素对免疫系统的调节机理作一综述,为家畜应激反应中糖皮质激素的免疫调节功能及其作用机制的研究提供思路和启发。
1 糖皮质激素对免疫功能调控的途径 1.1 糖皮质激素受体的特征糖皮质激素对免疫功能的调控主要是通过糖皮质激素受体(GR)实现的,糖皮质激素受体属于核转录因子,是核受体超家族成员之一,正常生理条件下,GR对糖皮质激素不敏感,只有在高浓度糖皮质激素的刺激下GR可被激活[12]。GRα和GRβ是GR的两个亚型,广泛分布于机体各种组织细胞中[13]。GRα分布于机体大部分的组织器官和免疫细胞中,GRβ主要存在于T细胞、巨噬细胞等免疫细胞中[14],在大多数细胞中GRα的含量远大于GRβ。GRα可结合糖皮质激素并激活相关应答基因表达,但GRβ不仅不能结合任何形式的糖皮质激素,同时也不具有激活转录表达的作用。在缺少配体的情况下,GR一般驻留在细胞质中,其与热休克蛋白和免疫亲和素等结合以伴侣蛋白分子的形式存在[15],当与配体结合后,伴侣蛋白分子复合体结构发生改变,并将GR释放,GR进入细胞核对靶基因的转录进行调控[16],从而抑制多种促炎性细胞因子的基因表达,阻碍炎症应答信号通路[17]。GRα和GRβ在机体中的表达不仅存在组织特异性[18],同一类细胞在家畜不同的生长发育阶段也有差异[19],这可能是家畜维持机体内环境平衡和免疫系统稳定的一种调节机制。
1.2 糖皮质激素受体的调控反刍动物对应激较为敏感[17, 20],应激可导致反刍动物体内糖皮质激素的大量分泌,糖皮质激素可刺激GR表达量增加,并使GR磷酸化,从而通过GR对靶基因的转录进行正向或负向调控[21],同时GR与配体结合后,也可参与调控多种非基因调节的信号通路[22],如参与调节磷脂酰肌醇激酶和T细胞受体信号通路,从而调控相关细胞因子的分泌和免疫细胞活动[23]。A.O′Loughlin等[24]研究发现,犊牛断奶后1 d GRα的表达量较断奶前升高了3倍,并在7 d内保持2倍以上的高水平,断奶后白细胞介素1(IL-1)、白细胞介素8(IL-8)、肿瘤坏死因子-α(TNF-α)、干扰素-γ(IFN-γ)、细胞凋亡因子(Fas)和Toll样受体4(TLR4) 的基因表达量显著上调2倍以上,表明应激可诱导炎症反应同时激活糖皮质激素调节机制。另一方面,应激引起的促炎性细胞因子分泌可影响GRα和GRβ的转录(表 1)。
|
|
表 1 促炎性细胞因子对GRα和GRβ的影响 Table 1 Effects of proinflammatory factors on the GRα and GRβ |
促炎性细胞因子可上调GRα的表达量,但IL-1通过激活促分裂原活化蛋白激酶抑制GRα调控的信号通路[25],导致靶细胞的GR对糖皮质激素不敏感[26-29]。此外,促炎性细胞因子可显著上调GRβ的表达[30-35],当GRα过表达时,GRβ可与GRα结合形成异源二聚体,产生竞争性抑制作用,干扰GRα的正常功能,这可能是糖皮质激素抵抗[1]和负反馈调节中的重要作用机制[36-37]。有关GRβ生物学功能还有待于进一步研究。
2 糖皮质激素对免疫细胞的调控机理 2.1 巨噬细胞单核细胞发生外渗作用时转变为巨噬细胞,在固有免疫中发挥关键作用,并可启动适应性免疫调节机制。发生炎症时微生物模式识别受体可激活巨噬细胞,同时巨噬细胞可释放多种促炎性细胞因子,启动抗原呈递信号通路等免疫活动,增强机体免疫功能(图 1)。在以小鼠为模型的研究中发现,糖皮质激素可通过诱导T细胞辅助细胞向Th2细胞分化来增加白细胞介素10(IL-10) 的分泌,从而抑制巨噬细胞的激活[17]。另一方面,糖皮质激素可通过某些束缚机制以及形成GR二聚体的方式抑制巨噬细胞功能,增强抗炎细胞因子的转录,抑制促炎性细胞因子的释放,从而抑制炎症反应[39-40]。在以小鼠为模型的研究中发现,糖皮质激素抑制巨噬细胞功能的束缚机制可能与干扰素调节因子-3(IRF-3) 和核转录因子κB (NF-κB)的基因表达被抑制有关[41],该机制还有待于在反刍动物上进行验证。研究表明,发生断奶应激时犊牛血液中单核细胞数较稳定[20, 24, 42-44]。另有研究表明,反刍动物血液中单核细胞数几乎不受运输应激的影响[45]。与其他动物相比,应激条件下糖皮质激素对反刍动物单核细胞的调控十分有限,目前尚不明确糖皮质激素水平的升高是否对反刍动物单核细胞的外渗有抑制作用,另有学者认为反刍动物的单核细胞存在阻碍快速响应糖皮质激素调节的免疫机制,故表现出对应激不敏感[46],但糖皮质激素影响巨噬细胞成熟和凋亡的机制还不明确,其调控巨噬细胞免疫功能的信号通路还有待进一步研究。
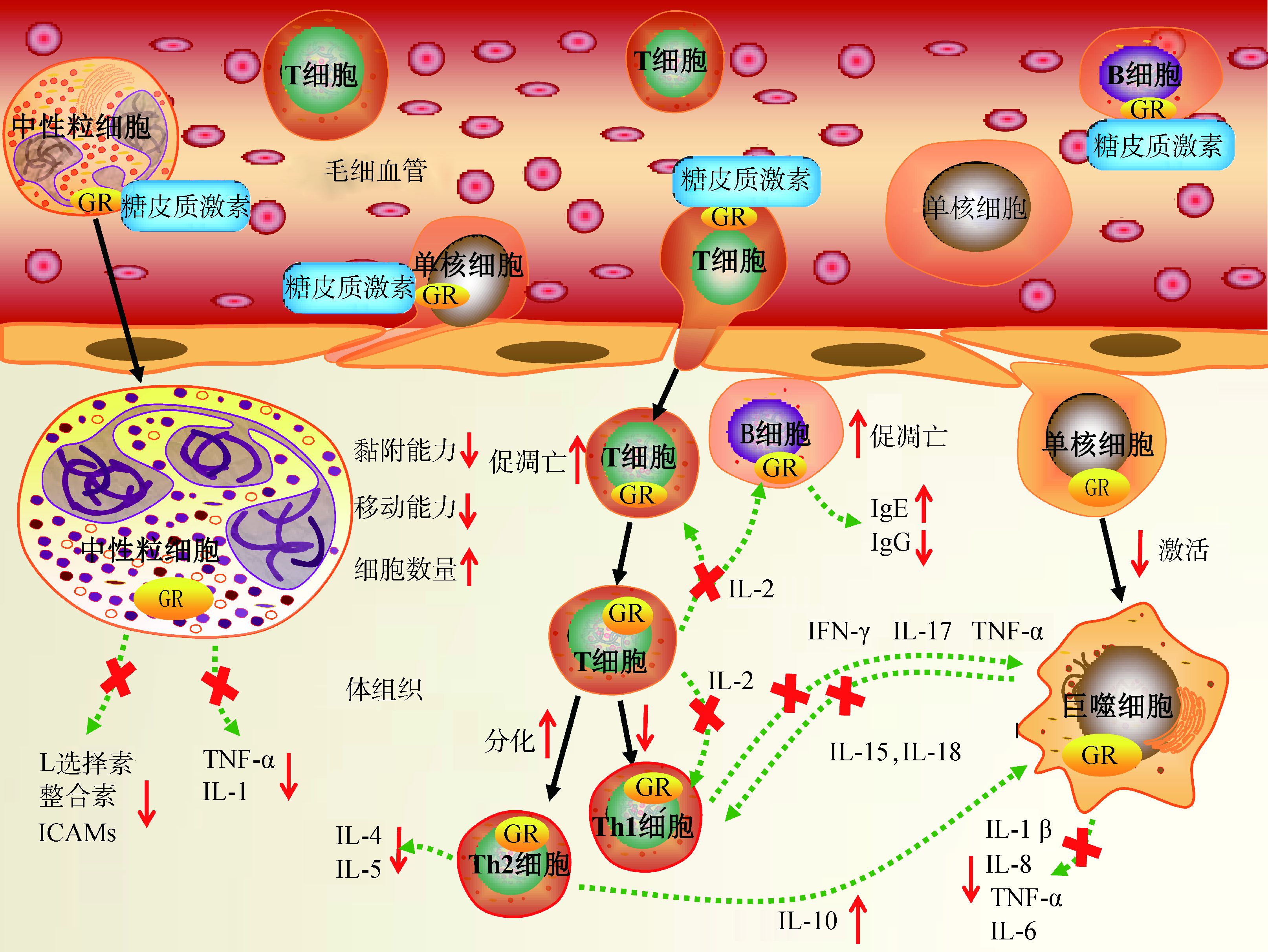
|
图 1 糖皮质激素受体对主要免疫细胞的调控 Figure 1 Effects of the glucocorticoid receptor on major immune cells |
中性粒细胞是应激引发炎症反应中的主要响应细胞(图 1),同时也是适应性免疫的关键识别效应细胞[47-48]。中性粒细胞不仅可以直接吞噬细菌、异物颗粒和衰亡细胞,还可以迅速释放多种细胞因子和趋化因子,对固有免疫和适应性免疫都具有调节功能[49-50]。研究表明,应激引起的糖皮质激素水平的升高会抑制家畜血液中细胞黏附分子L选择素(CD62L)的分泌,导致中性粒细胞的黏附和游走能力下降,减轻炎症浸润性组织反应的同时限制了中性粒细胞发挥其免疫学功能[42, 51]。此外,糖皮质激素还可通过抑制内皮细胞的白细胞整合素和细胞间黏附分子(ICAMs)的合成等途径来限制中性粒细胞的活动,阻碍其向炎症组织迁移,抑制中性粒细胞的免疫功能[52-54]。另一方面,应激条件下糖皮质激素水平升高可导致外周血液中的中性粒细胞数量异常增多。E.M.Lynch等[43]研究发现,断奶应激会导致犊牛血液中中性粒细胞数由断奶前的2.5×103个·μL-1升高至断奶后的4.2×103个·μL-1,并在断奶后3 d内显著高于断奶前水平。其原因可能是糖皮质激素通过某种机制延缓血液中中性粒细胞的衰亡[55],同时通过上调IL-8的表达量诱导骨髓中干细胞分化,导致血液中的中性粒细胞数增多[44]。虽然糖皮质激素水平的升高可上调血液中的中性粒细胞数,但会降低中性粒细胞的迁移和黏附能力,使其不能发挥正常的免疫学功能,从而抑制了家畜的免疫系统功能,增加患病风险。
2.3 淋巴细胞淋巴细胞尤其是T细胞在适应性免疫中起着关键作用。应激发生时糖皮质激素可通过抑制其前体细胞在胸腺成熟和诱导淋巴细胞凋亡的途径,使血液中淋巴细胞的数量降低[56],从而抑制家畜的免疫系统功能(图 1)。E.M.Lynch等[43]研究发现,断奶应激导致犊牛血液中淋巴细胞数由断奶前的7.3×103个·μL-1下降到断奶后的5.2×103个·μL-1,并在14 d内保持较低水平。M.C.Hickey等[57]和M.H.Kim等[58]也得到了类似的研究结果。目前,糖皮质激素对B细胞功能影响的研究较少,其主要影响B细胞前体细胞的增殖能力,免疫球蛋白E的分泌量增加,同时抑制免疫球蛋白G的合成,导致脾和淋巴结中B细胞数量减少[59-60]。糖皮质激素可诱导T细胞辅助细胞向Th2型分化,Th1细胞的减少可影响T细胞、自然杀伤细胞(NK)和巨噬细胞的激活,使其不能对炎症反应做出应答[61]。CD4+和CD8+T细胞在胸腺中的成熟由T细胞受体(TCR)信号通路调控,糖皮质激素的升高可明显抑制TCR信号通路,改变CD4+和CD8+T细胞比例的同时通过GR二聚体激活细胞凋亡程序引起胸腺细胞和未成熟的T细胞的过早凋亡[62-65],对家畜免疫系统造成损害,增加动物患病风险。目前,在犊牛上的研究表明,无论是大剂量或小剂量(2 mg vs 0.4 mg、5 mg vs 0.4 mg)的糖皮质激素都会对胸腺的发育造成损害并诱导胸腺细胞的过早凋亡[66-67]。发生应激时糖皮质激素水平升高对淋巴细胞的促凋亡作用以及对其免疫功能的抑制作用会影响家畜的健康,这一点值得关注。
3 糖皮质激素对细胞因子的调控 3.1 糖皮质激素对前炎性细胞因子的抑制糖皮质激素调控免疫系统功能的主要机制是通过调节靶细胞的核转录实现的,通过GR与糖皮质激素反应元件(GREs)相互作用调控核内NF-κB和激活蛋白1(AP1) 信号通路,从而阻断炎症应答。研究表明,应激犊牛接受糖皮质激素处理后,体内促炎性细胞因子IL-1、IL-6、IFN-γ和TNF-α的基因表达量会被抑制数小时[68]。另有研究表明,巨噬细胞和T细胞在接受糖皮质激素处理后,促炎性细胞因子TNF-α、IL-1β、白细胞介素2(IL-2)、白细胞介素6(IL-6) 的分泌明显受到抑制[39]。其机理可能是糖皮质激素通过激活GR抑制Th1细胞中靶基因的转录,从而抑制了巨噬细胞和T细胞的免疫信号通路[61]。另一方面,糖皮质激素可通过诱导Th2细胞的分化上调白细胞介素5(IL-5)、白细胞介素4(IL-4) 和白细胞介素10(IL-10) 等细胞因子的合成,从而抑制Th1细胞应答和巨噬细胞的抗原呈递功能以及IFN-γ等促炎性细胞因子的分泌[69]。此外,糖皮质激素对反刍动物免疫细胞因子的调控还存在如IRF等其他信号通路,其作用机理还有待于进一步研究。
3.2 糖皮质激素的致炎作用糖皮质激素水平的升高可上调Toll样受体(TLR)在多种细胞中的基因表达[70-71],且糖皮质激素可增强靶细胞对TNF-α和IFN-γ的敏感性,从而进一步刺激Toll样受体的分泌,这与糖皮质激素的抗炎作用相矛盾[70]。D.Rozkova等[70]研究发现,糖皮质激素可显著上调树突细胞中TLR2和TLR4的表达量,表明糖皮质激素存在致炎作用,但具体作用机理目前尚不清楚。综上所述,一方面糖皮质激素可通过激活GR抑制靶细胞的NF-κB和AP1通路,抑制促炎性细胞因子的分泌[72-74],可在细胞水平上抑制免疫细胞功能,从而减轻炎症反应,起到抗炎作用。另一方面,糖皮质激素可上调Toll样受体的基因表达,诱导炎症反应。在今后反刍动物的相关研究中,应注意糖皮质激素有抗炎作用的同时还有致炎作用。
3.3 糖皮质激素对细胞凋亡的调控糖皮质激素可通过TCR信号通路以及形成GR二聚体的方式诱导反刍动物的免疫细胞凋亡,阻碍其对炎症的免疫应答[62-65]。在炎症组织中糖皮质激素可阻断细胞凋亡的信号通路,阻碍炎症部位的细胞凋亡,但机制并不是通过GR直接调控靶细胞内的Fas,而是通过阻断其配体(FasL)的基因表达来实现[75-77],故在研究中可观察到应激条件下Fas的表达量升高数倍[24],实际上,在炎症组织中的细胞凋亡可能是被抑制的。目前在反刍动物应激反应的研究中,糖皮质激素通过调控靶细胞内FasL的表达量来影响细胞凋亡的调控途径并未引起重视,其对炎症组织中细胞凋亡的抑制作用可能会导致炎症和感染的扩散,损害动物健康。
4 小结反刍动物发生应激时糖皮质激素分泌量增加并激活靶细胞中的GR,通过抑制NF-κB和AP1通路,减少促炎性细胞因子的分泌,抑制免疫细胞功能,发挥其抗炎作用的同时对家畜免疫系统产生抑制。此外,糖皮质激素有促免疫细胞凋亡的作用,但其可能导致炎症组织中的细胞凋亡被抑制,增加了炎症和感染扩散的风险。另一方面,糖皮质激素还有致炎作用,可干扰免疫系统功能。目前,在反刍动物中对糖皮质激素调控免疫系统功能的研究十分有限,今后需通过对其作用机制和信号通路进行系统的研究,为通过营养调控手段缓解应激,增强家畜免疫功能和保障动物健康提供科学依据。
| [1] | OAKLEY R H, CIDLOWSKI J A. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids[J]. J Biol Chem, 2011, 286(5): 3177–3184. DOI: 10.1074/jbc.R110.179325 |
| [2] |
田青, 王洪荣, 王梦芝. 氢化可的松对奶牛乳腺上皮细胞酪蛋白合成的影响[J]. 畜牧兽医学报, 2014, 45(10): 1663–1670.
TIAN Q, WANG H R, WANG M Z. Effects of HYD on the synthesis of casein in mammary epithelial cells of holstein cows in vitro[J]. Acta Veterinaria et Zootechnica Sinica, 2014, 45(10): 1663–1670. (in Chinese) |
| [3] | REICHARDT H M, HORSCH K, GRONE H, et al. Mammary gland development and lactation are controlled by different glucocorticoid receptor activities[J]. Eur J Endocrinol, 2001, 145(4): 519–527. DOI: 10.1530/eje.0.1450519 |
| [4] | OLLIER S, BEAUDOIN F, VANACKER N, et al. Effect of reducing milk production using a prolactin-release inhibitor or a glucocorticoid on metabolism and immune functions in cows subjected to acute nutritional stress[J]. J Dairy Sci, 2016, 99(12): 9949–9961. DOI: 10.3168/jds.2016-11711 |
| [5] | GLASER R, KIECOLT-GLASER J K. Stress-induced immune dysfunction: implications for health[J]. Nat Rev Immunol, 2005, 5(3): 243–251. DOI: 10.1038/nri1571 |
| [6] | RIVIER C, VALE W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo[J]. Endocrinology, 1983, 113(3): 939–942. DOI: 10.1210/endo-113-3-939 |
| [7] | WATABE T, TANAKA K, KUMAGAE M, et al. Role of endogenous arginine vasopressin in potentiating corticotropin-releasing hormone-stimulated corticotropin secretion in man[J]. J Clin Endocrinol Metab, 1988, 66(6): 1132–1137. DOI: 10.1210/jcem-66-6-1132 |
| [8] |
李玮, 刘蕤, 马尧, 等. 重度冷应激对三河牛血液生化指标及相关基因表达的影响[J]. 畜牧兽医学报, 2015, 46(8): 1463–1470.
LI W, LIU R, MA R, et al. Effect of severe cold stress on blood biochemical parameters and related gene expression in sanhe cattle[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(8): 1463–1470. (in Chinese) |
| [9] | EVANS R M. The steroid and thyroid hormone receptor superfamily[J]. Science, 1988, 240(4854): 889–895. DOI: 10.1126/science.3283939 |
| [10] | CHINENOV Y, ROGATSKY I. Glucocorticoids and the innate immune system: crosstalk with the toll-like receptor signaling network[J]. Mol Cell Endocrinol, 2007, 275(1-2): 30–42. DOI: 10.1016/j.mce.2007.04.014 |
| [11] | DUFF G C, GALYEAN M L. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle[J]. J Anim Sci, 2007, 85(3): 823–840. DOI: 10.2527/jas.2006-501 |
| [12] | FELDMAN S, WEIDENFELD J. Electrical stimulation of the dorsal hippocampus caused a long lasting inhibition of ACTH and adrenocortical responses to photic stimuli in freely moving rats[J]. Brain Res, 2001, 911(1): 22–26. DOI: 10.1016/S0006-8993(01)02538-0 |
| [13] | CALDENHOVEN E, LIDEN J, WISSINK S, et al. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids[J]. Mol Endocrinol, 1995, 9(4): 401–412. |
| [14] | PUJOLS L, MULLOL J, TORREGO A, et al. Glucocorticoid receptors in human airways[J]. Allergy, 2004, 59(10): 1042–1052. DOI: 10.1111/all.2004.59.issue-10 |
| [15] | PRATT W B, TOFT D O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery[J]. Exp Biol Med, 2003, 228(2): 111–133. DOI: 10.1177/153537020322800201 |
| [16] | DEJAGER L, VANDEVYVER S, PETTA I, et al. Dominance of the strongest: inflammatory cytokines versus glucocorticoids[J]. Cytokine Growth Factor Rev, 2014, 25(1): 21–33. DOI: 10.1016/j.cytogfr.2013.12.006 |
| [17] | BASCHANT U, TUCKERMANN J. The role of the glucocorticoid receptor in inflammation and immunity[J]. J Steroid Biochem Mol Biol, 2010, 120(2-3): 69–75. DOI: 10.1016/j.jsbmb.2010.03.058 |
| [18] | TURNER J D, SCHOTE A B, MACEDO J A, et al. Tissue specific glucocorticoid receptor expression, a role for alternative first exon usage[J]. Biochem Pharmacol, 2006, 72(11): 1529–1537. DOI: 10.1016/j.bcp.2006.07.005 |
| [19] | PERLMAN W R, WEBSTER M J, HERMAN M M, et al. Age-related differences in glucocorticoid receptor mRNA levels in the human brain[J]. Neurobiol Aging, 2007, 28(3): 447–458. DOI: 10.1016/j.neurobiolaging.2006.01.010 |
| [20] | JOHNSTON D, KENNY D A, KELLY A K, et al. Characterisation of haematological profiles and whole blood relative gene expression levels in Holstein-Friesian and Jersey bull calves undergoing gradual weaning[J]. Animal, 2016, 10(9): 1547–1556. DOI: 10.1017/S1751731115002438 |
| [21] | NEWTON R, LEIGH R, GIEMBYCZ M A. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases[J]. Pharmacol Ther, 2010, 125(2): 286–327. DOI: 10.1016/j.pharmthera.2009.11.003 |
| [22] | HARR M W, RONG Y P, BOOTMAN M D, et al. Glucocorticoid-mediated inhibition of Lck modulates the pattern of T cell receptor-induced calcium signals by down-regulating inositol 1, 4, 5-trisphosphate receptors[J]. J Biol Chem, 2009, 284(46): 31860–31871. DOI: 10.1074/jbc.M109.005579 |
| [23] | LWENBERG M, STAHN C, HOMMES D W, et al. Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands[J]. Steroids, 2008, 73(9-10): 1025–1029. DOI: 10.1016/j.steroids.2007.12.002 |
| [24] | O'LOUGHLIN A, MCGEE M, WATERS S M, et al. Examination of the bovine leukocyte environment using immunogenetic biomarkers to assess immunocompetence following exposure to weaning stress[J]. BMC Vet Res, 2011, 7: 45. DOI: 10.1186/1746-6148-7-45 |
| [25] | WANG X, WU H, MILLER A H. Interleukin 1α (IL-1α) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function[J]. Mol Psychiatry, 2004, 9(1): 65–75. DOI: 10.1038/sj.mp.4001339 |
| [26] | SALEM S, HARRIS T, MOK J S L, et al. Transforming growth factor-β impairs glucocorticoid activity in the A549 lung adenocarcinoma cell line[J]. Br J Pharmacol, 2012, 166(7): 2036–2048. DOI: 10.1111/j.1476-5381.2012.01885.x |
| [27] | HU A H, JOSEPHSON M B, DIENER B L, et al. Pro-asthmatic cytokines regulate unliganded and ligand-dependent glucocorticoid receptor signaling in airway smooth muscle[J]. PLoS One, 2013, 8(4): e60452. DOI: 10.1371/journal.pone.0060452 |
| [28] | KEENAN C R, MOK J S L, HARRIS T, et al. Bronchial epithelial cells are rendered insensitive to glucocorticoid transactivation by transforming growth factor-β1[J]. Respir Res, 2014, 15: 55. DOI: 10.1186/1465-9921-15-55 |
| [29] | PAN X Y, WANG Y, SU J, et al. The mechanism and significance of synergistic induction of the expression of plasminogen activator inhibitor-1 by glucocorticoid and transforming growth factor beta in human ovarian cancer cells[J]. Mol Cell Endocrinol, 2015, 407: 37–45. DOI: 10.1016/j.mce.2015.03.005 |
| [30] | KAM J C, SZEFLER S J, SURS W, et al. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids[J]. J Immunol, 1993, 151(7): 3460–3466. |
| [31] | NIMMAGADDA S R, SZEFLER S J, SPAHN J D, et al. Allergen exposure decreases glucocorticoid receptor binding affinity and steroid responsiveness in atopic asthmatics[J]. Am J Respir Crit Care Med, 1997, 155(1): 87–93. DOI: 10.1164/ajrccm.155.1.9001294 |
| [32] | WEBSTER J C, OAKLEY R H, JEWELL C M, et al. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance[J]. Proc Natl Acad Sci U S A, 2001, 98(12): 6865–6870. DOI: 10.1073/pnas.121455098 |
| [33] | ORII F, ASHIDA T, NOMURA M, et al. Quantitative analysis for human glucocorticoid receptor α/β mRNA in IBD[J]. Biochem Biophys Res Commun, 2002, 296(5): 1286–1294. DOI: 10.1016/S0006-291X(02)02030-2 |
| [34] | STRICKLAND I, KISICH K, HAUK P J, et al. High constitutive glucocorticoid receptor β in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids[J]. J Exp Med, 2001, 193(5): 585–594. DOI: 10.1084/jem.193.5.585 |
| [35] | WANG Z L, LI P, ZHANG Q H, et al. Interleukin-1β regulates the expression of glucocorticoid receptor isoforms in nasal polyps in vitro via p38 MAPK and JNK signal transduction pathways[J]. J Inflamm, 2015, 12: 3. DOI: 10.1186/s12950-014-0046-z |
| [36] | SMOAK K A, CIDLOWSKI J A. Mechanisms of glucocorticoid receptor signaling during inflammation[J]. Mech Ageing Dev, 2004, 125(10-11): 697–706. DOI: 10.1016/j.mad.2004.06.010 |
| [37] | KINO T, SU Y A, CHROUSOS G P. Human glucocorticoid receptor isoform β: recent understanding of its potential implications in physiology and pathophysiology[J]. Cell Mol Life Sci, 2009, 66(21): 3435–3448. DOI: 10.1007/s00018-009-0098-z |
| [38] | TORREGO A, PUJOLS L, ROCA-FERRER J, et al. Glucocorticoid receptor isoforms alpha and β in vitro cytokine-induced glucocorticoid insensitivity[J]. Am J Respir Crit Care Med, 2004, 170(4): 420–425. DOI: 10.1164/rccm.200308-1143OC |
| [39] | REICHARDT H M, TUCKERMANN J P, GÖTTLICHER M, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor[J]. EMBO J, 2001, 20(24): 7168–7173. DOI: 10.1093/emboj/20.24.7168 |
| [40] | TUCKERMANN J P, KLEIMAN A, MORIGGL R, et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy[J]. J Clin Invest, 2007, 117(5): 1381–1390. DOI: 10.1172/JCI28034 |
| [41] | OGAWA S, LOZACH J, BENNER C, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors[J]. Cell, 2005, 122(5): 707–721. DOI: 10.1016/j.cell.2005.06.029 |
| [42] | LYNCH E M, EARLEY B, MCGEE M, et al. Effect of abrupt weaning at housing on leukocyte distribution, functional activity of neutrophils, and acute phase protein response of beef calves[J]. BMC Vet Res, 2010, 6: 39. DOI: 10.1186/1746-6148-6-39 |
| [43] | LYNCH E M, MCGEE M, DOYLE S, et al. Effect of pre-weaning concentrate supplementation on peripheral distribution of leukocytes, functional activity of neutrophils, acute phase protein and behavioural responses of abruptly weaned and housed beef calves[J]. BMC Vet Res, 2012, 8: 1. DOI: 10.1186/1746-6148-8-1 |
| [44] | O'LOUGHLIN A, MCGEE M, DOYLE S, et al. Biomarker responses to weaning stress in beef calves[J]. Res Vet Sci, 2014, 97(2): 458–463. DOI: 10.1016/j.rvsc.2014.06.003 |
| [45] | GUPTA S, EARLEY B, CROWE M A. Effect of 12-hour road transportation on physiological, immunological and haematological parameters in bulls housed at different space allowances[J]. Vet J, 2007, 173(3): 605–616. DOI: 10.1016/j.tvjl.2006.03.002 |
| [46] | JONES M L, ALLISON R W. Evaluation of the ruminant complete blood cell count[J]. Vet Clin North Am Food Anim Pract, 2007, 23(3): 377–402. DOI: 10.1016/j.cvfa.2007.07.002 |
| [47] | BORREGAARD N. Neutrophils, from marrow to microbes[J]. Immunity, 2010, 33(5): 657–670. DOI: 10.1016/j.immuni.2010.11.011 |
| [48] | AMULIC B, CAZALET C, HAYES G L, et al. Neutrophil function: from mechanisms to disease[J]. Annu Rev Immunol, 2012, 30(1): 459–489. DOI: 10.1146/annurev-immunol-020711-074942 |
| [49] | ROSALES C, DEMAUREX N, LOWELL C A, et al. Neutrophils: their role in innate and adaptive immunity[J]. J Immunol Res, 2016, 2016: 1469780. |
| [50] | JAILLON S, GALDIERO M R, PRETE D D, et al. Neutrophils in innate and adaptive immunity[J]. Semin Immunopathol, 2013, 35(4): 377–394. DOI: 10.1007/s00281-013-0374-8 |
| [51] | WEBER P S D, TOELBOELL T, CHANG L C, et al. Mechanisms of glucocorticoid-induced down-regulation of neutrophil L-selectin in cattle: evidence for effects at the gene-expression level and primarily on blood neutrophils[J]. J Leukoc Biol, 2004, 75(5): 815–827. |
| [52] | PITZALIS C, PIPITONE N, PERRETTI M. Regulation of leukocyte-endothelial interactions by glucocorticoids[J]. Ann N Y Acad Sci, 2002, 966: 108–118. DOI: 10.1111/nyas.2002.966.issue-1 |
| [53] | CRONSTEIN B N, KIMMEL S C, LEVIN R I, et al. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1[J]. Proc Natl Acad Sci U S A, 1992, 89(21): 9991–9995. DOI: 10.1073/pnas.89.21.9991 |
| [54] | PITZALIS C, PIPITONE N, BAJOCCHI G, et al. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: an additional mechanism for their anti-inflammatory and immunosuppressive effect[J]. J Immunol, 1997, 158(10): 5007–5016. |
| [55] | HEASMAN S J, GILES K M, WARD C, et al. Glucocorticoid-mediated regulation of granulocyte apoptosis and macrophage phagocytosis of apoptotic cells: implications for the resolution of inflammation[J]. J Endocrinol, 2003, 178(1): 29–36. DOI: 10.1677/joe.0.1780029 |
| [56] | RODRIGUES-MASCARENHAS S, SANTOS N F D, RUMJANEK V M. Synergistic effect between ouabain and glucocorticoids for the induction of thymic atrophy[J]. Biosci Rep, 2006, 26(2): 159–169. DOI: 10.1007/s10540-006-9012-1 |
| [57] | HICKEY M C, DRENNAN M, EARLEY B. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production[J]. J Anim Sci, 2003, 81(11): 2847–2855. DOI: 10.2527/2003.81112847x |
| [58] | KIM M H, YANG J Y, UPADHAYA S D, et al. The stress of weaning influences serum levels of acute-phase proteins, iron-binding proteins, inflammatory cytokines, cortisol, and leukocyte subsets in Holstein calves[J]. J Vet Sci, 2011, 12(2): 151–157. DOI: 10.4142/jvs.2011.12.2.151 |
| [59] | CUPPS T R, EDGAR L C, THOMAS C A, et al. Multiple mechanisms of B cell immunoregulation in man after administration of in vivo corticosteroids[J]. J Immunol, 1984, 132(1): 170–175. |
| [60] | CUPPS T R, GERRARD T L, FALKOFF R J, et al. Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation[J]. J Clin Invest, 1985, 75(2): 754–761. DOI: 10.1172/JCI111757 |
| [61] | MOSMANN T R, COFFMAN R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties[J]. Annu Rev Immunol, 1989, 7(1): 145–173. DOI: 10.1146/annurev.iy.07.040189.001045 |
| [62] | PAZIRANDEH A, XUE Y T, PRESTEGAARD T, et al. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis[J]. FASEB J, 2002, 16(7): 727–729. |
| [63] | FRAKER P J, KING L E. Reprogramming of the immune system during zinc deficiency[J]. Annu Rev Nutr, 2004, 24(1): 277–298. DOI: 10.1146/annurev.nutr.24.012003.132454 |
| [64] | BOMMHARDT U, BEYER M, HÜNIG T, et al. Molecular and cellular mechanisms of T cell development[J]. Cell Mol Life Sci, 2004, 61(3): 263–280. DOI: 10.1007/s00018-003-3224-3 |
| [65] | HEROLD M J, MCPHERSON K G, REICHARDT H M. Glucocorticoids in T cell apoptosis and function[J]. Cell Mol Life Sci, 2006, 63(1): 60–72. DOI: 10.1007/s00018-005-5390-y |
| [66] | BIOLATTI B, CANNIZZO F T, ZANCANARO G, et al. Effects of low-dose dexamethasone on thymus morphology and immunological parameters in veal calves[J]. J Vet Med A Physiol Pathol Clin Med, 2005, 52(4): 202–208. DOI: 10.1111/jva.2005.52.issue-4 |
| [67] | CANNIZZO F T, MINISCALCO B, RIONDATO F, et al. Effects of anabolic and therapeutic doses of dexamethasone on thymus morphology and apoptosis in veal calves[J]. Vet Rec, 2008, 163(15): 448–452. DOI: 10.1136/vr.163.15.448 |
| [68] | CARROLL J A, ARTHINGTON J D, CHASE C C. Early weaning alters the acute-phase reaction to an endotoxin challenge in beef calves[J]. J Anim Sci, 2009, 87(12): 4167–4172. DOI: 10.2527/jas.2009-2016 |
| [69] | FARRAR J D, OUYANG W J, LÖHNING M, et al. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3[J]. J Exp Med, 2001, 193(5): 643–650. DOI: 10.1084/jem.193.5.643 |
| [70] | ROZKOVA D, HORVATH R, BARTUNKOVA J, et al. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors[J]. Clin Immunol, 2006, 120(3): 260–271. DOI: 10.1016/j.clim.2006.04.567 |
| [71] | HOMMA T, KATO A, HASHIMOTO N, et al. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells[J]. Am J Respir Cell Mol Biol, 2004, 31(4): 463–469. DOI: 10.1165/rcmb.2004-0161OC |
| [72] | DOYLE S L, O'NEILL L A J. Toll-like receptors: from the discovery of NFκB to new insights into transcriptional regulations in innate immunity[J]. Biochem Pharmacol, 2006, 72(9): 1102–1113. DOI: 10.1016/j.bcp.2006.07.010 |
| [73] | GRIVENNIKOV S I, KUPRASH D V, LIU Z G, et al. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: from simple paradigms to complex mechanisms[J]. Int Rev Cytol, 2006, 252: 129–161. DOI: 10.1016/S0074-7696(06)52002-9 |
| [74] | SCHEINMAN R I, COGSWELL P C, LOFQUIST A K, et al. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids[J]. Science, 1995, 270(5234): 283–286. DOI: 10.1126/science.270.5234.283 |
| [75] | JU S T, PANKA D J, CUI H L, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation[J]. Nature, 1995, 373(6513): 444–448. DOI: 10.1038/373444a0 |
| [76] | BRUNNER T, MOGIL R J, LAFACE D, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas[J]. Nature, 1995, 373(6513): 441–444. DOI: 10.1038/373441a0 |
| [77] | DHEIN J, WALCZAK H, BÄUMLER C, et al. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95)[J]. Nature, 1995, 373(6513): 438–441. DOI: 10.1038/373438a0 |



