2. 铜仁学院乌江学院, 铜仁 554300;
3. 山西农业大学动物科技学院, 太谷 030801
2. Wujiang College, Tongren University, Tongren 554300, China;
3. College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu 030801, China
雌性动物卵泡GCs增殖和激素分泌,调控动物卵泡发育并贯穿整个繁殖过程[1-2]。多数动物卵泡发育过程呈波样模式,如人、牛和猪,由一个短暂的FSH浓度波峰启动卵泡波,卵泡细胞开始增殖并促进卵泡的发育,在这个过程中,FSH对卵泡GCs增殖和E2分泌起关键调节作用[3-4];同时,体外培养试验发现,胰岛素作用于FSH诱导的猪GCs上促黄体生成素(Luteotropic hormone, LH)和人绒毛膜促性腺激素(Human chorionic gonadotrophin,HCG)受体,促进GCs增殖并影响卵泡发育[5]。本课题组在无血清条件下体外培养绵羊卵泡GCs,也证明胰岛素和FSH对GCs增殖和E2分泌具有重要作用[6]。为了明确胰岛素和FSH对单胎绵羊与多胎动物猪的影响机理是否一致,设计本试验来研究猪卵泡GCs增殖和E2分泌与胰岛素和FSH有无剂量依赖性,从而揭示胰岛素和FSH对卵泡发育的调控机理,进而为明确猪卵泡发育过程及动物生殖生理提供理论基础。
1 材料与方法 1.1 材料选择3头正常发情的健康大白猪组,屠宰后分别采集双侧卵巢,投入冰浴缓冲液(DPBS)中,实验室分离并修剪卵泡。
1.2 试验方法 1.2.1 基础培养液的配置本试验在MEMα基础培养液中加入各种卵泡颗粒细胞生长所需物质,添加的各物质含量:100 000 U·L-1青霉素,5 μL·L-1 IGF-I,0.1 g·L-1链霉素,1.0×10-6 mol·L-1雄烯二酮,1 mg·L-1两性霉素B,5 mg·L-1转铁蛋白,0.02 mol·L-1HEPES,40 μg·L-1亚硒酸钠,1 g·L-1BSA,1.1 mL·L-1NEAA,0.84 g·L-1NaHCO3,调节基础培养液pH至7.0~7.4。
1.2.2 胰岛素和FSH的配制用配好的培养液将1 000 μg·mL-1胰岛素稀释成100、10、1 ng·mL-1备用;将10 μg·mL-1 FSH保存液稀释成25、5、1 ng·mL-1备用。
1.2.3 颗粒细胞的收集选取直径5~8 mm的卵泡,投入盛有培养液的平皿中,眼科剪一分为二剪开,刮刀刮取GCs,1 400 r·min-1离心5 min,培养液重悬。
1.2.4 颗粒细胞的体外培养试验共分16组,每组3个重复。48孔板每孔加入基础培养液130 μL;每孔加入50 μL约10万个活细胞;分别加入10 μL不同浓度的胰岛素和FSH,使胰岛素终浓度分别为0、1、10、100 ng·mL-1,FSH的浓度分别为0、1、5、25 ng·mL-1,体系终体积为200 μL;5% CO2,37 ℃培养。
Long-term细胞培养过程中,需在2、4和6 d后进行换液,换液时注意镜检GCs培养情况并拍照。培养7 d后,GCs用胰酶消化液处理[7],用移液枪吸取180 μL的上清液打入离心管中,培养结束后,收集培养液用于E2测定。
1.2.5 活细胞计数培养结束后的细胞用移液枪反复吹打,将块状细胞团吹打均匀,台盼蓝染色60 s,血细胞计数板镜检并计算活细胞数。
1.2.6 雌激素测定培养液E2浓度检测采用Estradiol Elisa Kit试剂盒(BlueGene,BG公司),根据培养液体积进行适当倍数的稀释,按照试剂盒提供的标准品和操作步骤测定OD450 nm。绘制标准曲线并计算样品OD450 nm值相应的E2浓度,培养液最终E2浓度需乘以稀释倍数。
1.2.7 数据分析细胞计数和E2测定值采用3次重复“平均值±标准误”表示,Excel作图,并通过SPSS软件对各指标进行显著性分析。
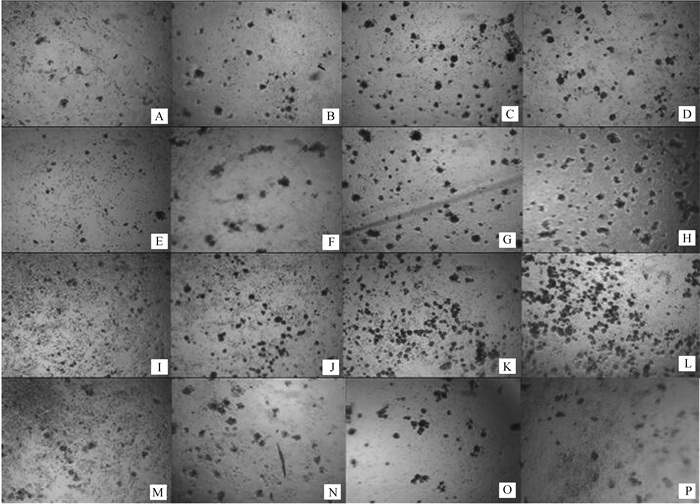
2 结果 2.1 卵泡GCs在胰岛素和FSH培养体系中体外培养7 d后显微图像GCs体外培养7 d后生长情况如图 1,当不添加胰岛素(图 1A、E、I、M),GCs随着FSH浓度的升高而增殖,增加到一定程度保持不变(图 1I、M);当培养液FSH浓度为0 ng·mL-1时(图 1A、B、C、D),胰岛素为100 ng·mL-1时细胞出现聚集形成团状(图 1D)。当FSH浓度为1 ng·mL-1(图 1E、F、G、H)和5 ng·mL-1(图 1I、J、K、L)时,GCs密度分别随胰岛素浓度的增加而增大;当FSH浓度为25 ng·mL-1(图 1M、N、O、P)时,胰岛素浓度的变化对GCs生长密度影响不大。所有培养体系中,当胰岛素浓度为100 ng·mL-1,FSH浓度为5 ng·mL-1时,GCs明显聚集成群,生长密度增大,数目达到最大值(图 1L),具体情况还需细胞计数验证。
|
A. 0 ng·mL-1 FSH, 0 ng·mL-1 insulin; B.0 ng·mL-1 FSH, 1 ng·mL-1 insulin; C.0 ng·mL-1FSH, 10 ng·mL-1 insulin; D. 0 ng·mL-1 FSH, 100 ng·mL-1 insulin; E.1 ng·mL-1 FSH, 0 ng·mL-1 insulin; F.1 ng·mL-1 FSH, 1 ng·mL-1 insulin; G.1 ng·mL-1 FSH, 10 ng·mL-1 insulin; H.1 ng·mL-1 FSH, 100 ng·mL-1 insulin; I.5 ng·mL-1 FSH, 0 ng·mL-1 insulin; J.5 ng·mL-1 FSH, 1 ng·mL-1 insulin; K.5 ng·mL-1 FSH, 10 ng·mL-1 insulin; L.5 ng·mL-1 FSH, 100 ng·mL-1 insulin; M.25 ng·mL-1 FSH, 0 ng·mL-1 insulin; N.0 ng·mL-1L FSH, 1 ng·mL-1 insulin; O.25 ng·mL-1 FSH, 10 ng·mL-1 insulin; P.25 ng·mL-1 FSH, 100 ng·mL-1 insulin 图 1 胰岛素和FSH培养体系中GCs 7 d后生长情况(10×10) Figure 1 The growth and development of follicular granulosa cells cultured for 7 d with adding different concentrations of insulin and FSH (10×10) |
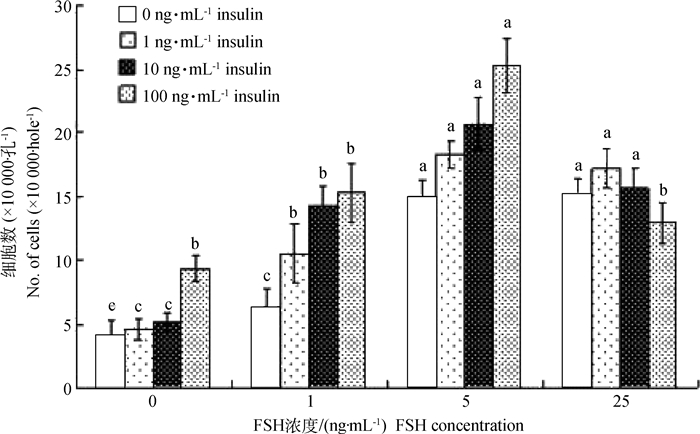
培养结束后细胞计数显示(图 2):当体系中胰岛素为0 ng·mL-1时,随着FSH浓度增加GCs数增多,且FSH浓度为5和25 ng·mL-1时,细胞数显著高于1 ng·mL-1和对照组(P < 0.05),但二者之间不存在显著差异,表明FSH对猪卵泡GCs的增殖有促进作用;当体系中FSH为0 ng·mL-1时,胰岛素浓度逐渐增加时细胞数增多,胰岛素达100 ng·mL-1时,细胞数最高,且显著高于其他不同浓度胰岛素各组(P < 0.05);由于体外培养过程中初始细胞密度为100 000·孔-1,即当培养体系中单独添加胰岛素并不能促进GCs增殖;当FSH为1 ng·mL-1时,胰岛素促GCs增殖的作用开始体现,与对照组(胰岛素为0 ng·mL-1)相比,其他各胰岛素组细胞密度均超过100 000·孔-1,且显著高于对照组(P < 0.05);当FSH为5 ng·mL-1时,胰岛素浓度的增加促进了GCs的增殖,但各胰岛素组间不存在显著差异(P>0.05),表明FSH是促进猪卵泡GCs增殖的主效因素;当FSH为25 ng·mL-1时,与FSH为5 ng·mL-1时相比较,GCs的增殖数下降。总之,通过对对照组(不添加FSH和胰岛素)活细胞计数,结果显示,GCs培养7 d后大量凋亡(初始细胞密度为100 000·孔-1),表明猪卵泡GCs的增殖对FSH有剂量依赖性。
|
字母代表 0.05水平显著性分析,字母相异差异显著,字母相同差异不显著,下同 Superscript small letters indicate significance at the level of 0.05. Values with the same letters are not significantly different and values with the different letters are significantly different. The same as follows 图 2 胰岛素和FSH培养体系中GCs 7 d后细胞计数 Figure 2 The number of granulosa cells after 7 d in vitro cultured at different concentrations of insulin and FSH |
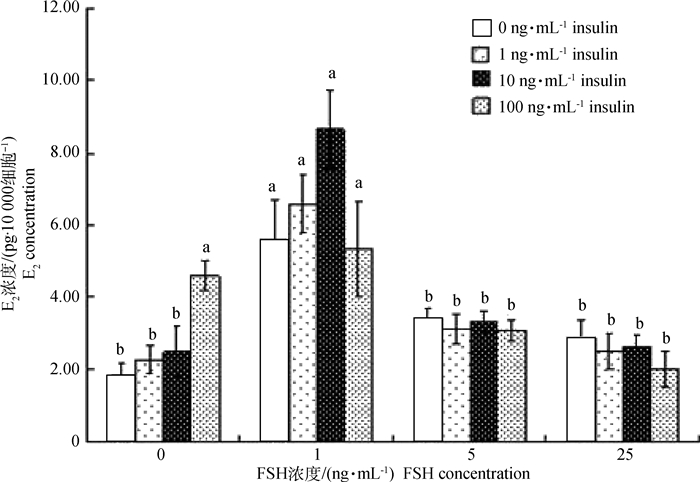
培养液E2测定结果显示(图 3):当体系中FSH为0 ng·mL-1时,胰岛素浓度逐渐增加时培养液E2浓度增高,当胰岛素达100 ng·mL-1时,E2分泌量最高,且显著高于其他各胰岛素组(P < 0.05);当体系中胰岛素为0 ng·mL-1时,FSH为1 ng·mL-1组的E2分泌量显著高于其它各组(P < 0.05),而其他各组间不存在显著差异,说明FSH诱导GCs分泌E2需在适当的FSH浓度范围内;当体系中FSH浓度为1 ng·mL-1时,胰岛素达10 ng·mL-1时的培养液E2浓度最高,但与其他不同胰岛素浓度组差异不显著,说明胰岛素和FSH在诱导猪卵泡GCs分泌E2的过程中,FSH作为主效因子发挥作用;同时,当体系中FSH为5和25 ng·mL-1时,胰岛素浓度的变化并不会影响培养液E2的浓度,这也进一步证明了猪卵泡发育过程中,FSH作为主效因子诱导GCs的增殖和E2的分泌,进而影响卵泡发育。
|
图 3 胰岛素和FSH培养体系中E2分泌水平 Figure 3 The E2 level in vitro culture at different concentrations of insulin and FSH |
动物卵泡生长发育的整个过程受到各种内分泌激素和卵泡内生长因子的调控,为了进一步揭示有腔卵泡生长发育的内分泌调控机制,近年来课题组一直致力于筛选和识别参与卵泡生长发育的局部调控分子。该研究通过Long-term体外试验探究在不同浓度胰岛素和FSH条件下,猪卵泡GCs体外增殖和E2分泌情况,深入分析胰岛素和FSH在猪卵泡发育过程中作用;结果表明,FSH在猪卵泡发育过程中发挥主要作用,且具有剂量依赖性;胰岛素具有促猪卵泡发育的作用,但必须在FSH诱导的情况下,才能显著促进GCs的增殖和E2的分泌。
卵泡生长发育过程中,E2浓度水平是反映卵泡生理状态重要的内分泌因子[8-9]。卵泡GCs体外培养研究表明,CART对颗粒层细胞E2分泌具有抑制作用[10-13],在卵泡发育期和黄体期,卵泡液E2水平较高,卵泡的发育与GCs增殖、GCs和TCs层中LH结合位点有关;相反,卵泡液E2水平较低,卵泡GCs中FSH结合位点数量较少[14-16]。卵泡发育过程中,FSH水平较低会导致从属卵泡(非优势卵泡)GCs中LIF-STAT3信号活性下降,是造成卵泡闭锁的的一个重要因素;FSH浓度水平提高则可恢复LIF-STAT3信号活性[17]。研究发现,在启动卵泡发育波前,高浓度FSH和LH的刺激可显著增强小卵泡的发育能力,但对原始卵泡募集不产生影响,持续增强刺激时间对卵泡的成熟和排卵具有显著效果[18]。本研究也获得相同的结果,适当浓度的FSH可显著提高GCs增殖和E2分泌。卵泡GCs膜上有特异性胰岛素受体分布,研究表明,胰岛素能促使FSH诱导猪GCs上LH/hCG受体,GCs培养液中加入胰岛素能显著增加孕酮分泌[4]。胰岛素也可与胰岛素样生长因子(Insulin-like growth factor, IGF)受体作用,促进细胞增殖,D.Schams[19]试验表明,IGF-1可显著提高猪和大鼠卵泡GCs增殖,对DNA的合成和GCs分化具有重要作用。M.Mihm等[20]对牛卵泡发育的试验中表明,IGFBP-4可显著提高GCs增殖和E2分泌,而高表达的IGFBP-2则促进卵泡GCs凋亡[21]。因此推测,适宜浓度的胰岛素可能促使IGFBP-4的表达量升高,过量的胰岛素,则使IGFBP-2表达量升高,这与本试验得出GCs数趋势基本一致,但要确定最适宜浓度,还需进一步探究。
B.Atanasov等[22]通过在牛生殖道孕酮(Progesterone,P)缓释装置研究表明,外源性P可显著提高牛排卵时血液P浓度并使排卵提前,同时显著增加了排卵卵泡直径和优势卵泡数量;通过药物刺激和适时断奶综合作用,其同期排卵率和卵泡发育进程显著提高,进而对受孕率产生影响[23]。E2和P浓度水平是影响动物卵泡发育的重要内分泌因子,在猪卵泡的发育过程中,卵泡发育阶段的划分不像牛和羊有成熟的理论作为依据,同时,对于哺乳动物卵泡颗粒层细胞分泌E2,而膜层细胞分泌P,因此,本试验也仅对GCs增殖和E2分泌情况进行了测定,用以表征胰岛素和FSH对猪卵泡发育的影响,该结果的产生及其机理有待于后期研究进一步论证。
在卵泡发育阶段,卵泡GCs开始生成FSH和LH的受体。GCs通过“双细胞-双促性腺激素模式”促进E2的合成,E2的形成能促进LH受体的分化和芳香化酶的合成,从而能促进E2进一步的合成。同时,下丘脑和垂体对促性腺激素的分泌具有调节作用,该调节功能有助于刺激动物卵泡发育[24]。体外培养人卵泡GCs的研究中,FSH的存在具有显著增强芳香化酶活性的作用,并与GCs增殖和E2分泌呈正相关[25-27]。本试验证实FSH促GCs增殖和E2分泌存在剂量效应关系,体系FSH为1 ng·mL-1组的E2分泌量显著高于5 ng·mL-1组和25 ng·mL-1组;培养体系中单独加入胰岛素或FSH,显微图像显示GCs生长不佳,甚至出现凋亡(GCs数低于原始细胞数量),培养液E2浓度也较低。因此,一定浓度的胰岛素或者与FSH互作均可提高GCs的增殖分化和E2的分泌[28],但培养液中高浓度的FSH反而会影响卵泡GCs合成E2的能力,这可能是由于高浓度的FSH导致卵泡细胞黄体化[29, 30],这也与本研究的结果相同。
4 结论猪卵泡GCs分泌E2需在FSH的诱导下产生,高浓度FSH会影响猪卵泡GCs的E2分泌;在适宜浓度下胰岛素能促进GCs增殖并提高E2的合成能力;二者在猪卵泡发育过程中发挥重要作用。
| [1] | LÜ L H, YUE W B, LIU W Z, et al. Effects of sperm capacitation treatments and in vitro culture systems on development of in vitro fertilized embryos derived from prepubertal boer goat oocytes[J]. Asian-Austral J Anim Sci, 2009, 22(7): 969–976. DOI: 10.5713/ajas.2009.90060 |
| [2] | LÜ L H, YUE W B, LIU W Z, et al. Effect of oocyte selection, estradiol and antioxidant treatment on in vitro maturation of oocytes collected from prepubertal Boer goats[J]. Ital J Anim Sci, 2010, 9(1): e11. DOI: 10.4081/ijas.2010.e11 |
| [3] | GINTHER O J, BASHIR S T, RAKESH H B, et al. Two-way coupling between FSH and the dominant follicle in heifers[J]. Theriogenology, 2013, 80(5): 463–469. DOI: 10.1016/j.theriogenology.2013.05.008 |
| [4] | BAERWALD A R, ADAMS G P, PIERSON R A. Characterization of ovarian follicular wave dynamics in women[J]. Biol Reprod, 2003, 69(3): 1023–1031. DOI: 10.1095/biolreprod.103.017772 |
| [5] | BOŁZAN E, ANDRONOWSKA A, BODEK G, et al. The novel effect of hCG administration on luteal function maintenance during the estrous cycle/pregnancy and early embryo development in the pig[J]. Pol J Vet Sci, 2013, 16(2): 323–332. |
| [6] |
李鹏飞, 岳文斌, 庞钰莹, 等. FSH和胰岛素对绵羊卵巢卵泡颗粒细胞体外培养的影响[J]. 畜牧兽医学报, 2013, 44(9): 1386–1391.
LI P F, YUE W B, PANG Y Y, et al. Effects of FSH and insulin on sheep ovarian follicular granulosa cells in vitro culture[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(9): 1386–1391. DOI: 10.11843/j.issn.0366-6964.2013.09.008 (in Chinese) |
| [7] | TORNER H, BRÜSSOW K P, ALM H, et al. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre-ovulatory maturation[J]. Theriogenology, 2004, 61(9): 1675–1689. DOI: 10.1016/j.theriogenology.2003.09.013 |
| [8] | EPPIG J J. Oocyte control of ovarian follicular development and function in mammals[J]. Reproduction, 2001, 122(6): 829–838. DOI: 10.1530/rep.0.1220829 |
| [9] | JUENGEL J L, HUDSON N L, HEATH D A, et al. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep[J]. Biol Reprod, 2002, 67(6): 1777–1789. DOI: 10.1095/biolreprod.102.007146 |
| [10] | SMITH S M, VAUGHAN J M, DONALDSON C J, et al. Cocaine-and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism[J]. Endocrinology, 2004, 145(11): 5202–5209. DOI: 10.1210/en.2004-0708 |
| [11] |
李鹏飞, 岳文斌, 黄洋, 等. 可卡因-苯丙胺调控转录肽对绵羊卵巢卵泡颗粒细胞雌激素产生的影响[J]. 畜牧兽医学报, 2013, 44(6): 853–857.
LI P F, YUE W B, HUANG Y, et al. Effects of CART on estradiol production in vitro in follicular granulose cells of sheep ovarian[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(6): 853–857. DOI: 10.11843/j.issn.0366-6964.2013.06.004 (in Chinese) |
| [12] |
李鹏飞, 岳文斌, 李富禄, 等. 可卡因-苯丙胺调控转录肽(CART)对猪卵巢卵泡颗粒细胞雌激素产生的影响[J]. 畜牧兽医学报, 2012, 43(12): 1879–1886.
LI P F, YUE W B, LI F L, et al. Effects of CART on estradiol production of pig ovarian follicular granulosa cells in vitro culture[J]. Acta Veterinaria et Zootechnica Sinica, 2012, 43(12): 1879–1886. (in Chinese) |
| [13] | SEN A, BETTEGOWDA A, JIMENEZ-KRASSEL F, et al. Cocaine-and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells[J]. Endocrinology, 2007, 148(9): 4400–4410. DOI: 10.1210/en.2007-0332 |
| [14] | IRELAND J J, ROCHE J F. Development of nonovulatory antral follicles in heifers:changes in steroids in follicular fluid and receptors for gonadotropins[J]. Endocrinology, 1983, 112(1): 150–156. DOI: 10.1210/endo-112-1-150 |
| [15] | IRELAND J J, ROCHE J F. Development of antral follicles in cattle after prostaglandin-induced luteolysis:changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors[J]. Endocrinology, 1982, 111(6): 2077–2086. DOI: 10.1210/endo-111-6-2077 |
| [16] | IRELAND J J, ROCHE J F. Growth and differentiation of large antral follicles after spontaneous luteolysis in heifers:changes in concentration of hormones in follicular fluid and specific binding of gonadotropins to follicles[J]. J Anim Sci, 1983, 57(1): 157–167. DOI: 10.2527/jas1983.571157x |
| [17] | ILHA G F, ROVANI M T, GASPERIN B G, et al. Lack of FSH support enhances LIF-STAT3 signaling in granulosa cells of atretic follicles in cattle[J]. Reproduction, 2015, 150(4): 395–403. DOI: 10.1530/REP-15-0026 |
| [18] | GARCÍA GUERRA A, TRIBULO A, YAPURA J, et al. Lengthened superstimulatory treatment in cattle:evidence for rescue of follicles within a wave rather than continuous recruitment of new follicles[J]. Theriogenology, 2015, 84(3): 467–476. DOI: 10.1016/j.theriogenology.2015.03.037 |
| [19] | SCHAMS D. Luteal peptides and intercellular communication[J]. J Reprod Fertil, 1987, 34: 87–99. |
| [20] | MIHM M, AUSTIN E J, GOOD T E M, et al. Identification of potential intrafollicular factors involved in selection of dominant follicles in heifers[J]. Biol Reprod, 2000, 63(3): 811–819. DOI: 10.1095/biolreprod63.3.811 |
| [21] | YUAN W, BAO B, GARVERICK H A, et al. Follicular dominance in cattle is associated with divergent patterns of ovarian gene expression for insulin-like growth factor (IGF)-Ⅰ, IGF-Ⅱ, and IGF binding protein-2 in dominant and subordinate follicles[J]. Domest Anim Endocrinol, 1998, 15(1): 55–63. DOI: 10.1016/S0739-7240(97)00062-3 |
| [22] | ATANASOV B, DE KOSTER J, BOMMELÉ L, et al. Pathways of the dominant follicle after exposure to sub-luteal circulating progesterone concentrations are different in lactating dairy cows versus non-lactating heifers[J]. Anim Reprod Sci, 2015, 154: 8–15. DOI: 10.1016/j.anireprosci.2015.01.002 |
| [23] | SILVA FILHO M L, BEZERRA L R, FERREIRA-SILVA J C, et al. Influence of biostimulation and temporary weaning on follicular dynamics and pregnancy rates in Nelore cows (Bos taurus indicus)[J]. Trop Anim Health Prod, 2015, 47(7): 1285–1291. DOI: 10.1007/s11250-015-0861-0 |
| [24] | RAK A, DRWAL E, KARPETA A, et al. Regulatory role of gonadotropins and local factors produced by ovarian follicles on in vitro resistin expression and action on porcine follicular steroidogenesis[J]. Biol Reprod, 2015, 92(6): 142. DOI: 10.1095/biolreprod.115.128611 |
| [25] | SEN A, LÜ L H, BELLO N, et al. Cocaine-and amphetamine-regulated transcript accelerates termination of follicle-stimulating hormone-induced extracellularly regulated kinase 1/2 and Akt activation by regulating the expression and degradation of specific mitogen-activated protein kinase phosphatases in bovine granulosa cells[J]. Mol Endocrinol, 2008, 22(12): 2655–2676. DOI: 10.1210/me.2008-0077 |
| [26] | LÜ L H, JIMENEZ-KEASSEL F, SEN A, et al. Evidence supporting a role for cocaine-and amphetamine-regulated transcript (CARTPT) in control of granulosa cell estradiol production associated with dominant follicle selection in cattle[J]. Biol Reprod, 2009, 81(3): 580–586. DOI: 10.1095/biolreprod.109.077586 |
| [27] | HUGHES Jr F M, GOROSPE W C. Biochemical identification of apoptosis (programmed cell death) in granulosa cells:evidence for a potential mechanism underlying follicular atresia[J]. Endocrinology, 1991, 129(5): 2415–2422. DOI: 10.1210/endo-129-5-2415 |
| [28] | SILVA J M, PRICE C A. Effect of follicle-stimulating hormone on steroid secretion and messenger ribonucleic acids encoding cytochromes P450 aromatase and cholesterol side-chain cleavage in bovine granulosa cells in vitro[J]. Biol Reprod, 2000, 62(1): 186–191. DOI: 10.1095/biolreprod62.1.186 |
| [29] | RAMAN R S, CHAN P J, CORSELLI J U, et al. Comet assay of cumulus cell DNA status and the relationship to oocyte fertilization via intracytoplasmic sperm injection[J]. Hum Reprod, 2001, 16(5): 831–835. DOI: 10.1093/humrep/16.5.831 |
| [30] | SHORES E M, PICTON H M, HUNTER M G. Differential regulation of pig theca cell steroidogenesis by LH, insulin-like growth factor I and granulosa cells in serum-free culture[J]. J Reprod Fertil, 2000, 118(2): 211–219. DOI: 10.1530/reprod/118.2.211 |



