2. 安徽地方畜禽遗传资源保护与生物育种省级实验室, 合肥 230036
2. Anhui Province Key Laboratory of Local Livestock and Poultry Genetic Resource Conservation and Bio-breeding, Hefei 230036, China
哺乳动物骨骼肌卫星细胞属于成体干细胞,位于骨骼肌质膜和基底膜之间[1]。当其活化后, 由静息通过增殖、分化为成肌细胞,再自我更新补充干细胞池或融合为新的肌纤维,从而影响骨骼肌形成、发育及其损伤后恢复过程[2]。这个过程受到内外因素的联合影响,不同外在因素通过信号通路介导卫星细胞的增殖和分化,同时,甲基化、microRNAs、组蛋白修饰等表观遗传学因素也参与到多种信号通路中[3]。Notch及P38 MAPK信号通路与卫星细胞的静息状态以及自我更新能力密切相关;Wnt及mTOR信号通路则抑制骨骼肌卫星细胞增殖并诱导其启动分化;JAK/STAT信号通路分别在增殖和分化期能引起卫星细胞不同特异性反应。多种信号通路相互联系形成精密的调控网络,共同实现骨骼肌的形成、发育及修复。本文概述了P38 MAPK、Notch、Wnt、mTOR、JAK/STAT等关键信号通路对卫星细胞增殖、分化和自我更新方面的作用机制和研究进展,对深入探究骨骼肌形成及肌肉发育相关疾病具有重要意义。
1 骨骼肌卫星细胞及其状态成体骨骼肌中,卫星细胞约占骨骼肌细胞总数的1%,且一般处于静息状态,但骨骼肌形成及损伤后修复取决于卫星细胞的存在。静息卫星细胞最可靠的转录因子Pax7及其同系物Pax3,能使胚胎干细胞定向的向肌源性祖细胞分化,在胚胎肌形成过程中起着关键作用,但其在出生后下调[4]。Pax7和Pax3是靶向上调促使卫星细胞增殖的基因,同时抑制其下游诱导肌源性分化的基因[5]。Pax转录因子的下游有负责卫星细胞向肌源性分化的调节因子MRFs,主要包括Myf5、MyoD、MyoG和Mrf4(也称为Myf6)[6]。MyoD蛋白表达是卫星细胞活化的标志,其转录活性增加可促进成肌分化,且其主要出现在卫星细胞增殖期[7]。
整个成肌分化途径包括静息卫星细胞的活化、增殖和分化为成肌细胞,融合形成肌管,并最终成熟为肌纤维(图 1)。静息的卫星细胞受到刺激后激活,并在大约24~36 h进入细胞周期进行有丝分裂[8]。部分卫星细胞进行不对称分裂,启动骨骼肌卫星细胞的增殖与分化。Pax7和Pax3分别通过直接结合MRFs的远端增强子元件和近端启动子来调节和诱导Myf5和MyoD的表达,Myf5和MyoD在肌卫星细胞中的表达量快速上调[9]。MyoG与Mrf4一起启动末端分化,细胞由扁圆形转变为梭形,融合为肌管,并成熟为肌纤维[10]。此外,卫星细胞对称分裂进行自我更新,在分裂后重新恢复静息状态以补充干细胞池,为此后肌肉修复损伤提供储备力量[11]。这些关键基因的顺序表达形成信号通路,与骨骼肌的增殖和分化有着密可不分的关系。
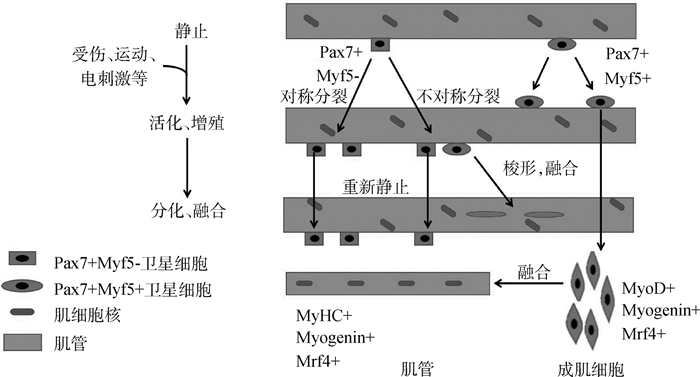
|
图 1 骨骼肌卫星细胞的增殖与分化 Figure 1 Process of skeletal muscle satellite cells proliferating and differentiating |
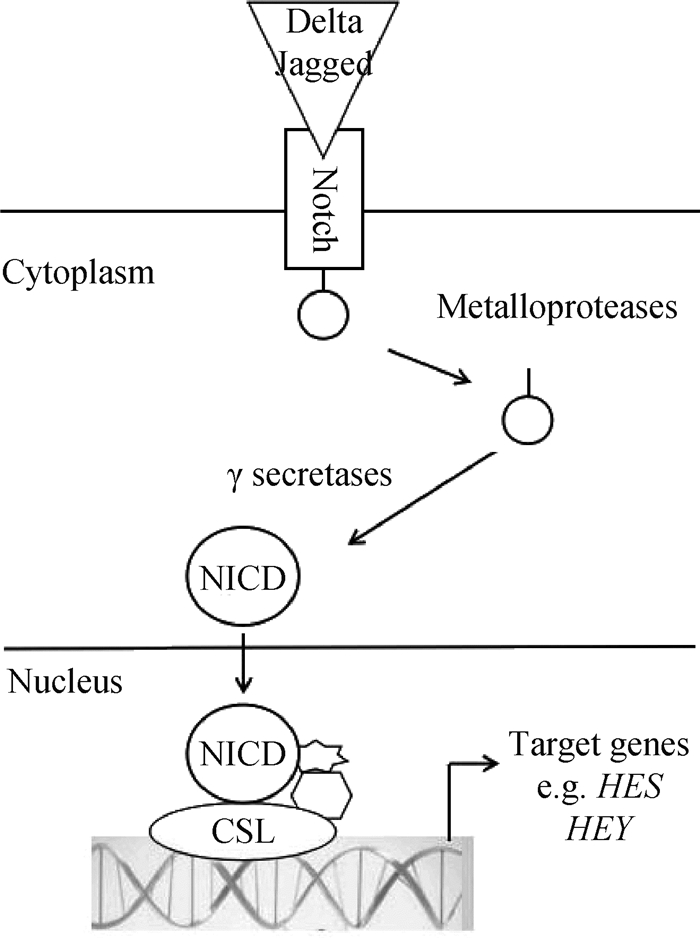
在哺乳动物中,Notch是一种编码膜蛋白受体,其信号通路由4种Notch受体(Notch1~4)、5种Notch配体(Dll1、Dll3、Dll4、Jagged1、Jagged2)以及细胞内效应器分子(Hes1、Hes5)3部分组成[12]。Notch信号通路活化被分为经典的Notch信号通路CSL(CBF-1/Suppressor of Hairless/LAG-1,又称RBP-Jκ)依赖途径和CSL非依赖途径2种。在经典Notch信号中,Notch受体与任一配体DSL (Delta/Serrate/LAG2)结合后使溶蛋白裂解,级联激活释放出Notch的胞内结构域(Notch intracellular domain,NICD),其进入细胞核与CSL蛋白结合,激活下游靶基因[13](图 2)。而通过非CSL依赖性途径时,Notch受体的ANK区和细胞内的锌指蛋白特异性结合,不需要Notch受体的裂解,也无需CSL参与[14]。
受体和配体的相互作用活化Notch信号通路,不同水平的Notch活化是卫星细胞自我更新和激活的开关。Y.F.Wen等[16]发现,特异性激活卫星细胞中的NICD可增强Pax7表达,过度加强骨骼卫星细胞的自我更新,可能导致肌肉再生不良。Notch通过Pten调控Akt信号促使FoxO1(Forkhead box protein O1)得到上调,同样促进骨骼肌卫星细胞的自我更新[17]。缺失Notch途径的转录共激活因子RBP-Jκ或受体Dll1产生卫星细胞的过早分化和消耗,丧失自我更新能力,引起出生后肌肉生长丧失和肌肉肌张力减退[18]。FoxO3促进Notch1和Notch3受体表达,使卫星细胞在自我更新过程中恢复静息状态[19]。Notch信号通路的激活促进肌肉卫星细胞静息和增殖,但抑制肌肉卫星细胞分化。Notch信号通过与Pax7上游2个结合位点(RBP-Jκ)的相互作用,来阻止卫星细胞的终末分化[20]。慢肌纤维中必要的转录因子Prox1在卫星细胞中能显著下调Notch1、Notch4、Nrarp、Dll4、Jag1,进而促进卫星细胞的分化[21]。用乙醇缺氧处理小鼠或敲除Lkb1能激活Notch信号通路,降低MyoD和MyoG表达,从而抑制细胞分化,同时上调Pax7水平[22]。Notch信号通路激活决定了pSmad3与细胞周期进程负调控物(如p15、p16、p21和p27等启动子)的结合能力。Notch和pSmad3相互拮抗控制卫星细胞增殖,同时也可控制肌肉干细胞的再生[23]。Notch1可能通过效应基因Hes5的激活,实现猪体内卫星细胞增殖并维持卫星细胞状态[24]。Notch信号通路还参与肌肉卫星细胞命运的抉择。Notch1信号的激活能诱导敲除Pax7的卫星细胞生成棕色脂肪,并且作为促进成年骨骼肌中棕色脂肪形成的分子开关[25]。这些研究表明,Notch信号通路在调节卫星细胞的命运以及骨骼肌发育中具有关键作用。出生后肌发生阶段,GSK-3β接连刺激Notch信号及经典Wnt信号通路,将卫星细胞增殖过渡到分化过程。Notch和Wnt信号之间的时间平衡协调肌肉前体细胞沿着肌原性谱系途径准确进展[26]。
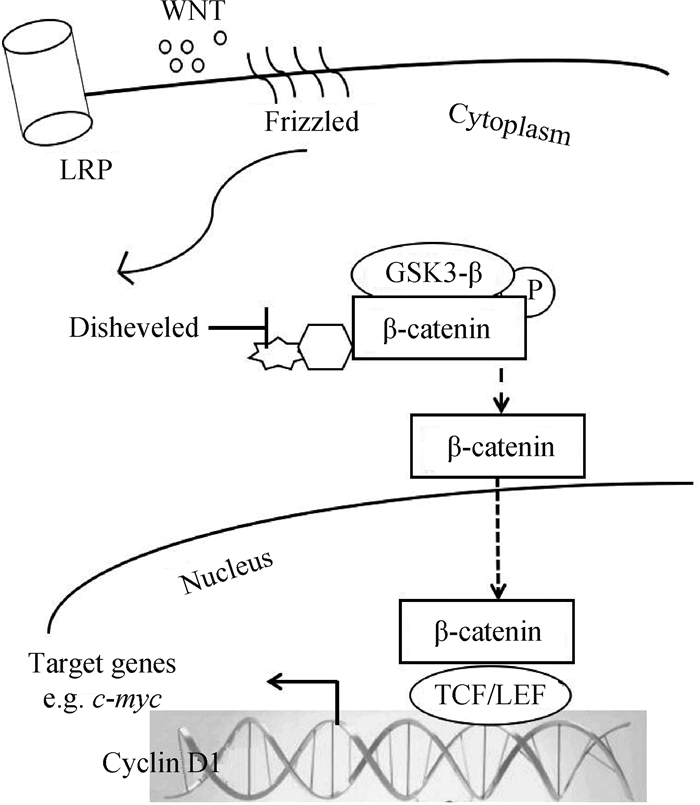
2.2 Wnt信号通路与骨骼肌卫星细胞的关系Wnt信号通路在多种组织中均存在,其分泌的信号分子家族具有高度的保守性,被认为是多细胞动物的第一次进化,在多细胞生物的出现过程中有着重要的作用[27]。在脊椎动物中,Wnt蛋白结合位于质膜中的卷曲蛋白(Frizzled,Fzd)受体上,也可在与LRP5/6结合后与Fzd形成三聚体复合物诱导多种细胞内反应[28]。
Wnt信号网主要包含3个途径,分别为Wnt/β-catenin、Wnt/Ca2+、Wnt/平面细胞极性(Planar-cell-polarity,PCP)。研究最广泛的为经典Wnt信号通路,主要涉及β-catenin/TCF/LEF转录复合物的激活。活化的Wnt蛋白与受体细胞Fzs及LRP5/6结合,其在生理条件下抑制GSK-3β活性,β-catenin与由axin、GSK-3β、APC组成的降解复合物结合的稳定性减弱,β-catenin的磷酸化降低,升高β-catenin浓度,继而转入细胞核内,并与TCF/LEF转录因子结合以促进靶基因的转录[29]。非经典的Wnt/Ca2 +、Wnt/PCP通路独立于β-catenin,直接与Fzd协调作用。Wnt与Fzd受体发生作用,激活GTP酶RhoA和Rac,诱导Jun靶基因,重塑细胞骨架;或增加Ca2+浓度,从而启动磷脂酶C(PLC)、蛋白激酶C(PKC)、CamKII和异源三聚体G蛋白引起的生物过程,同时能活化NFAT[30](图 3)。Wnt信号通路在胚胎和出生后骨骼肌发育中对细胞命运抉择和卫星细胞的增殖起到多种作用。
Wnt信号对胚胎细胞命运抉择及出生后早期骨骼肌发育卫星细胞的增殖具有不可替代的作用。在9.5胚龄小鼠胚胎中,Wnt1能诱导Myf5的表达,Wnt7a或Wnt6也能优先激活小鼠旁轴中胚层外植体培养物中的MyoD,从而诱导卫星细胞的成肌分化[31]。在经典Wnt/β-catenin通路中,信号转导对成年动物卫星细胞功能和骨骼肌再生的调节作用存在争议。A.Otto等[32]验证过度表达Wnt1、Wnt3a及Wnt5a使卫星细胞增殖显着增加,Wnt蛋白的激活可能调节骨骼肌再生期间诱导卫星细胞增殖的关键因素。M.M.Murphy等[33]则通过体内试验表明,不是激活而是沉默经典Wnt/β-catenin信号传导对成人肌肉再生有重要作用。最近研究显示,Wnt信号阻断成肌细胞显示延迟分化,有组成型活性β-catenin的成肌细胞经历早熟生长停滞和分化,Wnt/β-catenin活性在调节成体骨骼肌卫星细胞的增殖与分化中是必不可少的[34]。Wnt3、Wnt5a和Wnt5b在受到轻度刺激后的成年小鼠中显著上调,且TCF/LEF转录因子与MyoD和Myf5启动子区域具有可以结合的保守序列,也表明Wnt信号通路在促进骨骼肌卫星细胞的激活和分化中具有不可或缺的作用[27]。Wnt3a诱导Barx2表达,与β-catenin-TCF/LEF复合物结合调节Wnt靶基因如Axin2,从而上调卫星细胞的分化[35]。GSK-3β被Lkb1磷酸化,增加β-catenin浓度也能加速骨骼肌卫星细胞的分化[36]。利用LiCl作为激活剂能增加猪骨骼肌卫星细胞GSK-3β的表达量,加速促进猪肌卫星细胞的早期分化[37]。卫星干细胞Fzd7表达水平高,非经典通路Wnt7a/Fzd7/PCP启动卫星细胞的对称分裂,从而控制卫星细胞的内稳态水平[38]。Wnt7a/Fzd7信号的下游因子mTOR也能调节骨骼肌卫星细胞的增殖与肌纤维的分化[39]。
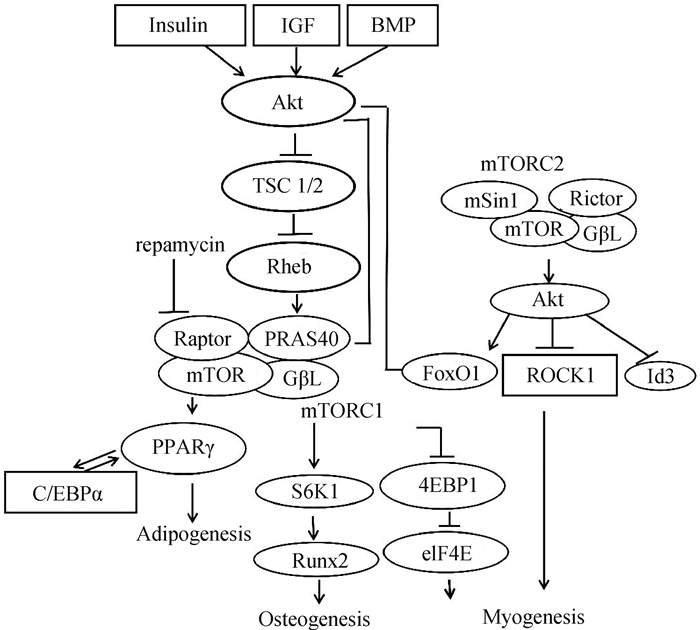
2.3 mTOR信号通路与骨骼肌卫星细胞的关系哺乳动物雷帕霉素靶点(Mammalian target of rapamycin,mTOR)是丝氨酸/苏氨酸蛋白激酶,主要作为mTOR复合物1(mTOR complex 1,mTORC1)和mTOR复合物2(mTOR complex 2,mTORC2)的催化亚基。mTOR通过其下游因子,如核糖体蛋白S6激酶(Ribosomal protein S6 kinase-1,S6k1)和eIF4-E结合蛋白1(eIF4-E binding protein 1,4E-BP1)结合mRNA,从而影响蛋白质合成和细胞生长速率[40](图 4)。
mTOR通过控制肌源性基因的表达调节骨骼肌卫星细胞的增殖和分化。转录因子Lbk1磷酸化激活AMPK/mTOR信号通路限制卫星细胞增殖[36]。小鼠卫星细胞敲除mTOR后,Pax7及MyoD表达水平显著下降,骨骼肌卫星细胞的增殖和分化均被抑制[41]。但近期有研究在小鼠中采用雷帕霉素抑制Ercc1-/Δ分离的骨骼肌卫星细胞mTOR信号通路,提高了骨骼肌卫星细胞肌原性分化能力,且凋亡和衰老细胞的百分比降低[42]。mTOR信号通路负调控整合素连锁激酶(ILK),上调MEF2C,进一步调节山羊肌肉卫星细胞中相关基因的表达[43]。骨骼肌肥大与卫星细胞的激活和分化息息相关,研究最多的是经典的PI3K/AKT/mTOR通路。胰岛素样生长因子1 (Insulin like growthfactor I,IGF-1)激活磷脂酰肌醇3激酶(Phosphat idylinositol 3 kinase,PI3K),从而产生Akt结合位点并激活mTOR,继而使S6k1和4E-BP1活化来增加蛋白质水平,使肌纤维肥大[45]。mTORC1的活化是处于休眠期G0卫星细胞激活所必不可少的因素[46]。另外,以渥曼青霉素(Wortmannin, WM)阻断PI3K/AKT信号通路会引起FoxO1去磷酸化,进而抑制猪骨骼肌卫星细胞的分化,降低成肌分化标志基因MyoG和MyHC的表达[47]。在绵羊卫星细胞中发现,mTORC1蛋白可通过调节S6k1负反馈作用于PI3K/Akt,继而促使骨骼肌肥大[48]。mTORC2虽不是骨骼肌发育所必要的,但最近有研究鉴定出一种新的Rictor/mTOR结合分子hnRNP M(Heterogeneous nuclear ribo nucleo protein M),其通过mTORC2信号使血清和糖皮质激素调节蛋白激酶(Serum/Glcocorticoid-regulated kinase 1,SGK1)磷酸化以调节肌肉的分化过程[49]。
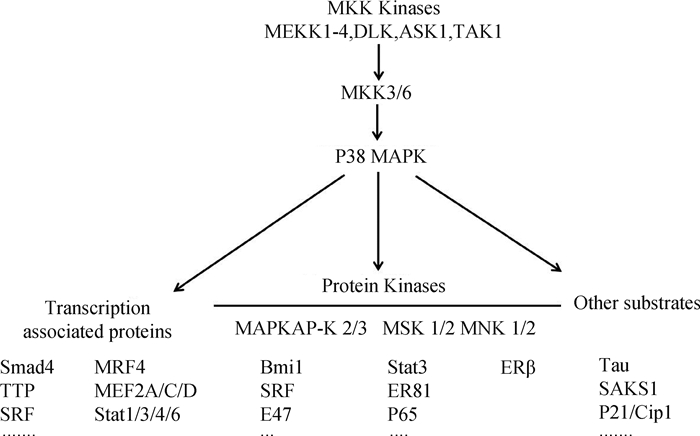
2.4 P38 MAPK信号通路与骨骼肌卫星细胞的关系P38 MAPK信号是骨骼肌卫星细胞增殖和分化的主要控制器之一[50]。丝裂原活化蛋白激酶(Mitogen activated protein kinases,MAPK)家族成员主要包括ERK 1/2、JNK/SAPK、P38MAPK、ERK5/BMK1以及非典型MAPK[51]。P38是MAPK家族控制炎症反应最重要的成员,哺乳动物中P38MAPK由4个成员(MAPK14(P38α)、MAPK11(P38β)、MAPK12(P38γ)和MAPK13(P38δ))组成。P38 MAPKs由上游MKK3/MKK6激活或磷酸化,活化的P38 MAPK进一步磷酸化下游的蛋白激酶和转录因子,调控多种基因的表达[52](图 5)。
研究表明,P38α/β MAPK具有促进人卫星细胞成肌分化,抑制移植卫星细胞增殖的能力,并可削弱部分成体卫星细胞的自我更新[54]。卫星细胞通过不对称分裂补充干细胞池,仅在一个子细胞中活化P38α/β MAPK,同时诱导MyoD增加,促使卫星细胞由静息转变为激活状态[55]。卫星细胞激活后,P38α/β的信号传导使三氚核苷酸(Tristetrapolin,TTP)失活,稳定MyoD mRNA以促进卫星细胞的分化过程[56]。敲除P38α的卫星细胞会分化延迟,导致小鼠出生后生长缺陷,使卫星细胞数量持续增加[57]。P38α不是直接作用于Pax7对卫星细胞增殖进行调控的,其经由Ezh2的磷酸化介导PRC2(Polycomb repressive complex 2)从而抑制Pax7启动子在肌肉干细胞分化过程中的表达[58-59]。P38α还通过上调JNK中磷酸酶MPK-1抑制促进增殖的JNK途径,导致细胞退出细胞周期开启卫星细胞的成肌分化[53]。沉默P38α会伴随P38γ磷酸化增加,激活的P38γ在Ser199和Ser200上直接磷酸化MyoD,使MyoD对肌细胞生成素启动子的占用率增加,并降低其转录活性[60]。
2.5 JAK/STAT信号通路与骨骼肌卫星细胞的关系除上述通路,JAK/STAT信号通路对卫星细胞的作用在近年来也逐渐被揭示。Janus激酶(Janus kinases,JAK)有4个家族,包括JAK1、JAK2、JAK3和TYK2。JAK有7个同源结构域(JAK homology domain, JH),定义为存在JH1和JH2两个相邻的激酶结构域。转录因子STAT(Signal transducer and activator of transcription)包含STAT1~4、STAT5A、STAT5B和STAT6等7个家族成员。当受体与配体结合产生构象变化,使受体偶联特异性酪氨酸残基磷酸化而活化JAKs。活化的JAKs产生募集信号转导物和转录激活物(STAT)的结合位点。最后,被活化的STAT形成二聚体转移到细胞核内与特异性启动子结合调控细胞增殖、分化和凋亡等转录活动[61](图 6)。
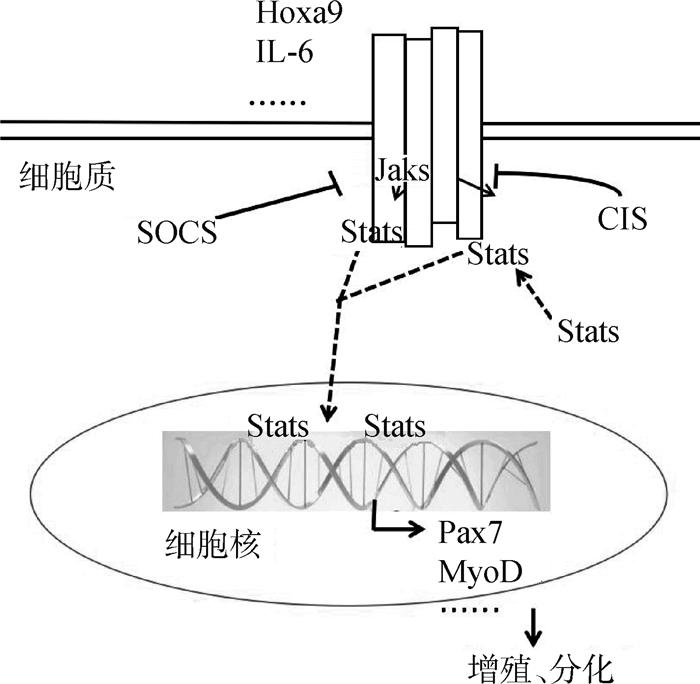
|
图 6 JAK/STAT信号通路 Figure 6 JAK/STAT signaling pathway |
JAK/STAT信号通路在肌发生的不同时期引起特异性反应。JAK1/STAT1/STAT3轴促使激活的卫星细胞增殖,防止过早分化成肌管。而JAK2/STAT2/STAT3途径介导MyoD和MEF2正调节卫星细胞分化。卫星细胞分化期间,JAK1和STAT1磷酸化水平降低,JAK2和STAT3磷酸化水平增高以抑制细胞的增殖[62]。激活JAK/STAT信号通路使老化卫星细胞缺乏再生能力,但小分子抑制剂处理JAK/STAT后,卫星细胞数目增加且再生能力明显增强[63]。M.T.Tierney等[64]发现,白细胞介素6(Interleukin-6,IL-6)通过调节STAT3从而控制卫星细胞的行为,使MyoD增加以促进卫星细胞的分化。利用不同小鼠模型揭示了在成体表达Pax7的卫星细胞中STAT3较低,增加了卫星细胞在肌肉再生期间的自我更新能力,但阻止了其在损伤后进行分化,造成肌肉损伤[65]。此外,使卫星细胞过表达Hoxa9且使用药理学原理抑制JAK/STAT可提高骨骼肌卫星细胞群的形成能力[66]。
3 骨骼肌卫星细胞中信号通路的研究展望 3.1 信号通路对骨骼肌卫星细胞作用的应用目前,诸如Notch、Wnt、P38 MAPK等多条重要信号通路对卫星细胞激活、增殖及分化的调控作用不断被揭示,为理解卫星细胞活动的分子机制提供了基本框架。成年肌肉中抑制Pax7表达的卫星细胞导致肌肉无法再生,表明卫星细胞可能是修复损伤后骨骼肌的唯一干细胞来源[67]。揭示卫星细胞相关信号通路,可在一定程度上了解胚胎成肌分化过程及成年动物肌肉损伤后的修复机制。组织工程技术的飞速发展,利用骨骼肌卫星细胞进行骨骼肌的再生可以避免以往用手术修复肌肉瘫痪的缺陷,为瘫痪肌肉的动力性修复开创了一个较新的研究前景。因此,阐述信号通路对卫星细胞的作用为理解动物肌肉形成过程与修复机制给予了理论支持,为组织工程技术及干细胞治疗提供了理论依据。总之,了解信号通路对卫星细胞增殖与分化的作用,为解决与肌细胞代谢及运动障碍相关疾病的研究奠定了基础,也为提高肉用家畜早期肌肉的形成,增强其市场竞争力,加速我国畜牧业持续健康的发展提供了参考。
3.2 骨骼肌卫星细胞中有关信号通路仍待深入研究的科学问题信号通路对动物骨骼肌卫星细胞的增殖与分化过程的调控相互交错,不同信号通路间彼此可以相互联系,相互作用,形成复杂的调控网络,进而调节卫星细胞活动。尽管有很多关于卫星细胞相关信号通路的研究,但各通路之间的相互影响机制尚不完全明了。也有各种研究表明,骨骼肌在受到外界环境变化刺激后卫星细胞活动开始发生变化,而变化发生后卫星细胞如何使肌纤维肌及干细胞建立新的稳态,信号通路所扮演的角色等问题仍然有待完善。信号通路可以调控卫星细胞的活动,但从卫星细胞的形成到肌细胞的肥大,其是否直接参与卫星细胞的启动,对卫星细胞增殖与分化的启动这一系列的生理生化过程的调控也尚不清晰。随着研究的进一步深入,信号通路对骨骼肌卫星细胞活动的调控机制将被揭示。
| [1] |
何波, 郑嵘, 熊远著, 等. 新生猪骨骼肌卫星细胞的培养鉴定及生物学特性[J]. 畜牧兽医学报, 2006, 37(6): 555–559.
HE B, ZHENG R, XIONG Y Z, et al. Culture, identification and biological characteristics of skeletal muscle satellite cells of the neonatal pig[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(6): 555–559. (in Chinese) |
| [2] | CHANG N C, RUDNICKI M A. Satellite cells:the architects of skeletal muscle[J]. Curr Top Dev Biol, 2014, 107: 161–181. DOI: 10.1016/B978-0-12-416022-4.00006-8 |
| [3] |
沈林園, 张顺华, 吴泽辉, 等. 骨骼肌卫星细胞对肉品质的影响及其分化调控[J]. 遗传, 2013, 35(9): 1081–1086.
SHEN L Y, ZHANG S H, WU Z H, et al. The influence of satellite cells on meat quality and its differential regulation[J]. Hereditas (Beijing), 2013, 35(9): 1081–1086. (in Chinese) |
| [4] | SHELTON M, METZ J, LIU J, et al. Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells[J]. Stem Cell Rep, 2014, 3(6): 1159. DOI: 10.1016/j.stemcr.2014.11.005 |
| [5] | SOLEIMANI V D, PUNCH V G, KAWABE Y I, et al. Transcriptional dominance of Pax7 in adult myogenesis is due to high-affinity recognition of homeodomain motifs[J]. Dev Cell, 2012, 22(6): 1208–1220. DOI: 10.1016/j.devcel.2012.03.014 |
| [6] | WANG C, WANG M, ARRINGTON J, et al. Ascl2 inhibits myogenesis by antagonizing the transcriptional activity of myogenic regulatory factors[J]. Development, 2017, 144(2): 235–347. DOI: 10.1242/dev.138099 |
| [7] | HWANG J, LEE S J, YOO M, et al. Kazinol-P from Broussonetia kazinoki, enhances skeletal muscle differentiation via p38MAPK and MyoD[J]. Biochem Biophys Res Commun, 2015, 456(1): 471–475. DOI: 10.1016/j.bbrc.2014.11.109 |
| [8] | WEBSTER M T, MANOR U, LIPPINCOTT-SCHWARTZ J, et al. Intravital imaging reveals ghost fibers as architectural units guiding myogenic progenitors during regeneration[J]. Cell Stem Cell, 2016, 18(2): 243–252. DOI: 10.1016/j.stem.2015.11.005 |
| [9] | GÜNTHER S, KIM J, KOSTIN S, et al. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells[J]. Cell Stem Cell, 2013, 13(5): 590–601. DOI: 10.1016/j.stem.2013.07.016 |
| [10] | RUDNICKI M A, LE GRAND F, MCKINNELL I, et al. The molecular regulation of muscle stem cell function[J]. Cold Spring Harb Sym Quant Biol, 2008, 73: 323–331. DOI: 10.1101/sqb.2008.73.064 |
| [11] | COLLINS C A, OLSEN I, ZAMMIT P S, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche[J]. Cell, 2005, 122(2): 289–301. DOI: 10.1016/j.cell.2005.05.010 |
| [12] | GREENWALD I, KOVALL R. Notch signaling:genetics and structure[J]. WormBook, 2013, 1(17): 1–28. |
| [13] | PREVIS R A, COLEMAN R L, HARRIS A L, et al. Molecular pathways:translational and therapeutic implications of the Notch signaling pathway in cancer[J]. Clin Cancer Res, 2015, 21(5): 955–961. DOI: 10.1158/1078-0432.CCR-14-0809 |
| [14] | ZHANG K J, LU Q Y, NIU X Q, et al. Notch1 signaling inhibits growth of EC109 esophageal carcinoma cells through downmodulation of HPV18 E6/E7 gene expression[J]. Acta Pharmacol Sin, 2009, 30(2): 153–158. DOI: 10.1038/aps.2008.16 |
| [15] | TSIVITSE S. Notch and Wnt signaling, physiological stimuli and postnatal myogenesis[J]. Int J Biol Sci, 2010, 6(3): 268–281. |
| [16] | WEN Y F, BI P P, LIU W Y, et al. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells[J]. Cell Mol Biol, 2012, 32(12): 2300–2311. DOI: 10.1128/MCB.06753-11 |
| [17] | BI P P, KUANG S H. Notch signaling as a novel regulator of metabolism[J]. Trends Endocrinol Metab, 2015, 26(5): 248–255. DOI: 10.1016/j.tem.2015.02.006 |
| [18] | YUE F, BI P P, WANG C, et al. Pten is necessary for the quiescence and maintenance of adult muscle stem cells[J]. Nat Commun, 2017, 8: 14328. DOI: 10.1038/ncomms14328 |
| [19] | GOPINATH S D, WEBB A E, BRUNET A, et al. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal[J]. Stem Cell Rep, 2014, 2(4): 414–426. DOI: 10.1016/j.stemcr.2014.02.002 |
| [20] | MOURIKIS P, GOPALAKRISHNAN S, SAMBASIVAN R, et al. Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells[J]. Development, 2012, 139(24): 4536–4548. DOI: 10.1242/dev.084756 |
| [21] | KIVELÄ R, SALMELA I, NGUYEN Y H, et al. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation[J]. Nat Commun, 2016, 7: 13124. DOI: 10.1038/ncomms13124 |
| [22] | SHAN T Z, ZHANG P P, XIONG Y, et al. Lkb1 deletion upregulates Pax7 expression through activating Notch signaling pathway in myoblasts[J]. Int J Biochem Cell Biol, 2016, 76: 31–38. DOI: 10.1016/j.biocel.2016.04.017 |
| [23] | CARLSON M E, HSU M, CONBOY I M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells[J]. Nature, 2008, 454(7203): 528–532. DOI: 10.1038/nature07034 |
| [24] | QIN L L, XU J, WU Z F, et al. Notch1-mediated signaling regulates proliferation of porcine satellite cells (PSCs)[J]. Cell Signal, 2013, 25(2): 561–569. DOI: 10.1016/j.cellsig.2012.11.003 |
| [25] | PASUT A, CHANG N C, GURRIARAN-RODRIGUEZ U, et al. Notch signaling rescues loss of satellite cells lacking Pax7 and promotes brown adipogenic differentiation[J]. Cell Rep, 2016, 16(2): 333–343. DOI: 10.1016/j.celrep.2016.06.001 |
| [26] | BRACK A S, CONBOY I M, CONBOY M J, et al. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis[J]. Cell Stem Cell, 2008, 2(1): 50–59. DOI: 10.1016/j.stem.2007.10.006 |
| [27] | FUJIMAKI S, HIDAKA R, ASASHIMA M, et al. Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running[J]. J Biol Chem, 2014, 289(11): 7399–7412. DOI: 10.1074/jbc.M113.539247 |
| [28] | CLEVERS H, NUSSE R. Wnt/β-catenin signaling and disease[J]. Cell, 2012, 149(6): 1192–1205. DOI: 10.1016/j.cell.2012.05.012 |
| [29] |
杨秋梅, 史新娥, 沈清武, 等. Wnt/β-catenin信号通路相关基因及MyHCs在猪骨骼肌发育中的表达规律[J]. 畜牧兽医学报, 2011, 42(12): 1686–1695.
YANG Q M, SHI X E, SHEN Q W, et al. The expression pattern of Wnt/β-catenin signal pathway related genes and MyHCs during porcine skeletal muscle development[J]. Acta Veterinaria et Zootechnica Sinica, 2011, 42(12): 1686–1695. (in Chinese) |
| [30] | VON MALTZAHN J, CHANG N C, BENTZINGER C F, et al. Wnt signaling in myogenesis[J]. Trends Cell Biol, 2012, 22(11): 602–609. DOI: 10.1016/j.tcb.2012.07.008 |
| [31] | TAJBAKHSH S, BORELLO U, VIVARELLI E, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5[J]. Development, 1998, 125(21): 4155–4162. |
| [32] | OTTO A, SCHMIDT C, LUKE G, et al. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration[J]. J Cell Sci, 2008, 121(17): 2939–2950. DOI: 10.1242/jcs.026534 |
| [33] | MURPHY M M, KEEFE A C, LAWSON J A, et al. Transiently active Wnt/β-catenin signaling is not required but must be silenced for stem cell function during muscle regeneration[J]. Stem Cell Rep, 2014, 3(3): 475–488. DOI: 10.1016/j.stemcr.2014.06.019 |
| [34] | RUDOLF A, SCHIRWIS E, GIORDANI L, et al. β-catenin activation in muscle progenitor cells regulates tissue repair[J]. Cell Rep, 2016, 15(6): 1277–1290. DOI: 10.1016/j.celrep.2016.04.022 |
| [35] | HULIN J A, NGUYEN T D T, CUI S, et al. Barx2 and Pax7 regulate Axin2 expression in myoblasts by interaction with β-catenin and chromatin remodelling[J]. Stem Cells, 2016, 34(8): 2169–2182. DOI: 10.1002/stem.2396 |
| [36] | SHAN T Z, ZHANG P P, LIANG X R, et al. Lkb1 is indispensable for skeletal muscle development, regeneration, and satellite cell homeostasis[J]. Stem Cells, 2014, 32(11): 2893–2907. DOI: 10.1002/stem.1788 |
| [37] |
杨映娟. Wnt/β-catenin信号通路对猪肌卫星细胞增殖分化影响的初步研究[D]. 杨凌: 西北农林科技大学, 2008.
YANG Y J. Primary research on the effects of Wnt/β-catenin signalling on pig satellite cells' proliferation and differentiation[D]. Yangling:Northwest A & F University, 2008. (in Chinese) http: //cdmd. cnki. com. cn/Article/CDMD-10712-2008101725. htm |
| [38] | LE GRAND F, JONES A E, SEALE V, et al. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells[J]. Cell Stem Cell, 2009, 4(6): 535–547. DOI: 10.1016/j.stem.2009.03.013 |
| [39] | VON MALTZAHN J, BENTZINGER C F, RUDNICKI M A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle[J]. Nat Cell Biol, 2012, 14(2): 186–191. |
| [40] | MA X M, BLENIS J. Molecular mechanisms of mTOR-mediated translational control[J]. Nat Rev Mol Cell Biol, 2009, 10(5): 307–318. DOI: 10.1038/nrm2672 |
| [41] | ZHANG P P, LIANG X R, SHAN T Z, et al. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration[J]. Biochem Biophys Res Commun, 2015, 463(1-2): 102–108. DOI: 10.1016/j.bbrc.2015.05.032 |
| [42] | TAKAYAMA K, KAWAKAMI Y, LAVASANI M, et al. mTOR signaling plays a critical role in the defects observed in muscle-derived stem/progenitor cells isolated from a murine model of accelerated aging[J]. J Orthop Res, 2017, 35(7): 1375–1382. DOI: 10.1002/jor.v35.7 |
| [43] | WU H Q, REN Y, PAN W, et al. The mammalian target of rapamycin signaling pathway regulates myocyte enhancer factor-2C phosphorylation levels through integrin-linked kinase in goat skeletal muscle satellite cells[J]. Cell Biol Int, 2015, 39(11): 1264–1273. DOI: 10.1002/cbin.v39.11 |
| [44] | XIANG X X, ZHAO J, XU G Y, et al. mTOR and the differentiation of mesenchymal stem cells[J]. Acta Biochim Biophys Sin, 2011, 43(7): 501–510. DOI: 10.1093/abbs/gmr041 |
| [45] | SCHIAFFINO S, DYAR K A, CICILIOT S, et al. Mechanisms regulating skeletal muscle growth and atrophy[J]. FEBS J, 2013, 280(17): 4294–4314. DOI: 10.1111/febs.2013.280.issue-17 |
| [46] | RODGERS J T, KING K Y, BRETT J O, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert[J]. Nature, 2014, 510(7505): 393–396. |
| [47] |
史新娥, 吴国芳, 宋子仪, 等. 阻断PI3K/AKT通路通过激活FoxO1抑制猪骨骼肌卫星细胞分化[J]. 中国农业科学, 2014, 47(1): 154–160.
SHI X E, WU G F, SONG Z Y, et al. Inhibition of PI3K/AKT pathway suppressing porcine skeletal muscle sattelite differentiation through activation of FoxO1 transcription factor[J]. Scientia Agricultura Sinica, 2014, 47(1): 154–160. (in Chinese) |
| [48] |
吴海青. mTOR信号通路对山羊骨骼肌卫星细胞增殖及分化的影响[D]. 呼和浩特: 内蒙古大学, 2015.
WU H Q. The effects of mammalian target of rapamycin signaling pathway on proliferation and differentiation of goat skeletal muscle satellite cells[D]. Hohhot:Inner Mongolia University, 2015. (in Chinese) http: //cdmd. cnki. com. cn/Article/CDMD-10126-1015361786. htm |
| [49] | CHEN W Y, LIN C L, CHUANG J H, et al. Heterogeneous nuclear ribonucleoprotein M associates with mTORC2 and regulates muscle differentiation[J]. Sci Rep, 2017, 7: 41159. DOI: 10.1038/srep41159 |
| [50] | SEGALÉS J, PERDIGUERO E, MUÑOZ-CÁNOVES P. Epigenetic control of adult skeletal muscle stem cell functions[J]. FEBS J, 2015, 282(9): 1571–1588. DOI: 10.1111/febs.2015.282.issue-9 |
| [51] | CARGNELLO M, ROUX P P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases[J]. Microbiol Mol Biol Rev, 2011, 75(1): 50–83. DOI: 10.1128/MMBR.00031-10 |
| [52] | ZARUBIN T, HAN J H. Activation and signaling of the p38 MAP Kinase pathway[J]. Cell Res, 2005, 15(1): 11–18. DOI: 10.1038/sj.cr.7290257 |
| [53] | PERDIGUERO E L, RUIZ-BONILLA V, SERRANO A L, et al. Genetic deficiency of p38α reveals its critical role in myoblast cell cycle exit:the p38α-JNK connection[J]. Cell Cycle, 2007, 6(11): 1298–1303. DOI: 10.4161/cc.6.11.4315 |
| [54] | CHARVILLE G W, CHEUNG T H, YOO B, et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells[J]. Stem Cell Rep, 2015, 5(4): 621–632. DOI: 10.1016/j.stemcr.2015.08.004 |
| [55] | TROY A, CADWALLADER A B, FEDOROV Y, et al. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38α/β MAPK[J]. Cell Stem Cell, 2012, 11(4): 541–553. DOI: 10.1016/j.stem.2012.05.025 |
| [56] | HAUSBURG M A, DOLES J D, CLEMENT S L, et al. Post-transcriptional regulation of satellite cell quiescence by TTP-mediated mRNA decay[J]. eLife, 2015, 4: e03390. |
| [57] | BRIEN P, PUGAZHENDHI D, WOODHOUSE S, et al. p38α MAPK regulates adult muscle stem cell fate by restricting progenitor proliferation during postnatal growth and repair[J]. Stem Cells, 2013, 31(8): 1597–1610. DOI: 10.1002/stem.1399 |
| [58] | MOZZETTA C, CONSALVI S, SACCONE V, et al. Selective control of Pax7 expression by TNF-activated p38α/polycomb repressive complex 2(PRC2) signaling during muscle satellite cell differentiation[J]. Cell Cycle, 2011, 10(2): 191–198. |
| [59] | JUAN A H, DERFOUL A, FENG X S, et al. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells[J]. Genes Dev, 2011, 25(8): 789–794. DOI: 10.1101/gad.2027911 |
| [60] | GILLESPIE M A, LE GRAND F, SCIMÈ A, et al. p38-γ-dependent gene silencing restricts entry into the myogenic differentiation program[J]. J Cell Biol, 2009, 187(7): 991–1005. DOI: 10.1083/jcb.200907037 |
| [61] | THOMAS S J, SNOWDEN J A, ZEIDLER M P, et al. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours[J]. Br J Cancer, 2015, 113(3): 365–371. DOI: 10.1038/bjc.2015.233 |
| [62] | TRENERRY M K, GATTA P A D, CAMERON-SMITH D. JAK/STAT signaling and human in vitro myogenesis[J]. BMC Physiol, 2011, 11(1): 6. DOI: 10.1186/1472-6793-11-6 |
| [63] | PRICE F D, VON MALTZAHN J, BENTZINGER C F, et al. Corrigendum:inhibition of JAK-STAT signaling stimulates adult satellite cell function[J]. Nat Med, 2014, 20(10): 1174–1181. DOI: 10.1038/nm.3655 |
| [64] | TIERNEY M T, AYDOGDU T, SALA D, et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair[J]. Nat Med, 2014, 20(10): 1182–1186. DOI: 10.1038/nm.3656 |
| [65] | ZHU H, XIAO F, WANG G, et al. STAT3 regulates self-renewal of adult muscle satellite cells during injury-induced muscle regeneration[J]. Cell Rep, 2016, 16(8): 2102–2115. DOI: 10.1016/j.celrep.2016.07.041 |
| [66] | SCHWÖRER S, BECKER F, FELLER C, et al. Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals[J]. Nature, 2016, 540(7633): 428–432. DOI: 10.1038/nature20603 |
| [67] | LEPPER C, PARTRIDGE T A, FAN C M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration[J]. Development, 2011, 138(17): 3639–3646. DOI: 10.1242/dev.067595 |